Boston University Nephrology Section has a long history of ground breaking basic and translational research in areas of glomerular disease, auto-immune disease, acute kidney injury, electrolyte disorders, uremic vascular disease, systemic amyloidosis, kidney cancer and cystic kidney disease, and kidney development and congenital kidney disease. For fellows interested in clinical research, research training can occur in the Waikar Lab, the Framingham Heart Study, BU School of Public Health, and BU amyloidosis center. Nephrology research laboratories are described in detail below.
Research training can occur under following mechanisms:
- Research pathway in a 2-year clinical nephrology fellowship
- Research fellowship under the NIH’s T32 research training program
This program requires fellows to possess either US citizenship or permanent residency. - Research fellowship outside of the NIH’s T32 research training program
Fellows with a strong research potential who do not have US Citizenship or permanent residency are mentored to apply for funding through foundations and professional societies in their first or second year of fellowship. We have a strong record of extra-mural funding for fellows through the National Kidney Foundation, the American Heart Association and the American Society of Nephrology. In the past, exceptional fellows have also been funded beyond their second year of fellowship using internal nephrology section funds.
Nephrology Research Laboratories:
Beck / Salant Lab
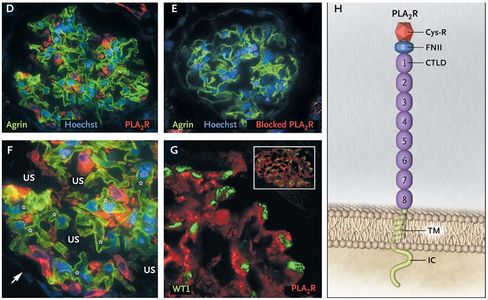
Beck / Salant laboratory explores the immune basis of glomerular diseases and the mechanisms of podocyte injury. Pioneering work from this lab led to the identification of PLA2R (phospholipase A2 receptor) as the major target antigen in membranous nephropathy in 2009 and THSD7A (thrombospondin type-1 domain-containing 7A) as a minor target antigen in membranous nephropathy in 2014. These discoveries are considered one of the most ground-breaking discoveries in nephrology in the last 20 years. In addition to basic research, this lab is also engaged in cutting-edge translational research using tissue samples and data from participants enrolled in the Nephrotic Syndrome Study Network (NEPTUNE) to better characterize molecular and genetic mechanisms of membranous nephropathy.
Five selected publications:
1. Yuan H et al. Nephrin dissociates from actin and its expression is reduced in early experimental membranous nephropathy. Journal of the American Society of Nephrology, 2002. PMID 11912254
2. Beck LH et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New England Journal of Medicine, 2009. PMID: 25394321
3. Beck LH et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. Journal of the American Society of Nephrology, 2012. PMID 21784898
4. Tomas NM and Beck LH et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. New England Journal of Medicine, 2014. PMID: 25394321
5. Larsen CP et al. LDL Receptor-Related Protein 2 (Megalin) as a Target Antigen in Human Kidney Anti-Brush Border Antibody Disease. Journal of the American Society of Nephrology, 2018, PMID: 29074737
Borkan Lab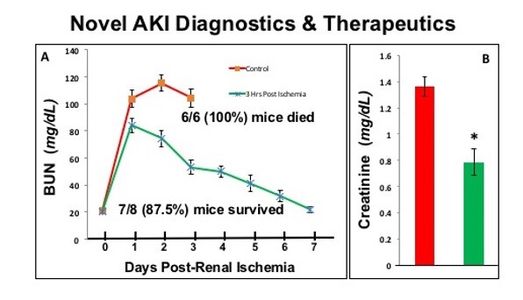
Borkan laboratory investigates pathways that promote renal cell survival and minimize acute kidney injury (AKI) after a variety of clinical insults, including ischemia and nephrotoxin exposure. The lab focuses on signaling pathways that mediate renal cell death in order to interrupt them and preserve organ function. The lab is currently investigating nucleophosmin, a link between rapid AKI diagnostics and peptide-based therapeutics that interrupt protein-protein interaction and significantly reduce AKI severity. The state-of-the art genetic screening and computational tools are used to identify AKI signaling pathways during nephrotoxic antibiotic exposure. We are currently funded by the NIH (RO-1 DK118267A1) to study “Nucleophosmin Centered Diagnostics and Treatment of Ischemic Acute Kidney Injury”.
Five selected publications:
1. Wang Z et al. Nucleophosmin, a critical Bax cofactor in ischemia-induced cell death. Molecular and Cellular Biology, 2013. PMID: 23459946
2. Gall JM et al. Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. Journal of the American Society of Nephrology, 2015. PMID: 25201884
3. Wang, Z et al. Nucleophosmin Phosphorylation as a Diagnostic and Therapeutic Target for Ischemic AKI. Journal of the American Society of Nephrology, 2019. PMID: 30573638
4. Igwebuike C et al. Cross Organelle Stress Response Disruption Promotes Gentamicin-Induced Proteotoxicity. Cell Death and Disease, 2020. PMID: 32245975
5. Wang et al. T95 Nucleophosmin Threonine 95 Phosphorylation as a Novel Mediator and Marker of Cell Death. American Journal of Physiology, 2020.
Chitalia Lab
Chitalia laboratory focuses on understanding the post-translational modifications of proteins and their role in kidney failure and cancer-related vascular pathologies. The lab harnesses the power of research tools spanning various cellular and molecular biological models, animal models (zebrafish and mice), mass spectrometry, genomic analysis, computational methods, and machine learning techniques to develop a highly integrative platform to understand the molecular mechanisms that drive disease pathogenesis. The lab intends to gain a deeper understanding of pathobiology to help develop a theranostic platform in future.
Five selected publications:
1. Chitalia VC et al. Matrix-embedded cells are protected from the uremic mileu. Nephrology Dialysis and Transplantation, 2011. PMID: 21795755
2. Chitalia VC et al. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation, 2013. PMID: 23269489
3. Shivanna S et al. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and antithrombotic target in uremia. Journal of the American Society of Nephrology, 2016. PMID: 26019318
4. Shashar M et al. Targeting STUB1-tissue factor axis normalizes hyerthrombotic uremic phenotype without increasing bleeding risk. Science Translational Medicine, 2017. PMID 29167396
5. Kolachalma VB et al. Uremic solute-aryl-hydrocarbon receptor-tissue factor axis associates with thrombosis and vascular injury in humans. Journal of the American Society of Nephrology, 2018. PMID: 29343519
Cohen Lab
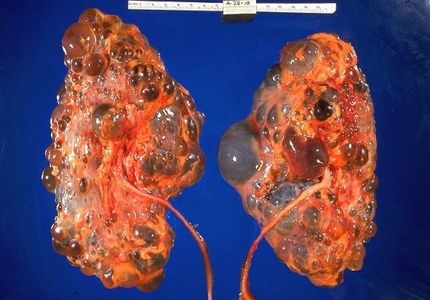
Cohen laboratory studies the molecular basis of renal cancer, renal cystic disease and renal development, and offers special expertise in gene expression mechanisms, signal transduction, protein-protein interactions, transcription factors, and renal epithelial cell biology. The laboratory has identified the first member of a new protein family, the Jade family of proteins. Jade-1 is a novel growth suppressive transcription factor that is stabilized by pVHL, a key component of the cellular oxygen sensing system. Jade 1 has important roles in autosomal dominant polycystic kidney disease (ADPKD), renal cancer, renal cyst formation, and renal development.
Five selected publications:
1. Zhou MI et al. Jade-1, a candidate renal tumor suppressor that promotes apoptosis. Proceeds of the National Academy of Sciences of the United States of America, 2005. PMID: 16046545
2. Cohen HT and McGovern FJ. Renal-cell carcinoma. New England Journal of Medicine, 2005. PMID: 16339066
3. Chitalia VC et al. Jade-1 inhibits Wnt signaling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL. Nature Cell Biology, 2008. PMID: 18806787
4. Foy RL et al. Polycystin-1 regulates the stability and ubiquitination of transcription factor Jade-1. Human Molecular Genetics, 2012. PMID: 23001567
5. Zheng L et al. Candidate tumor suppressor and pVHL partner Jade-1 binds and inhibits AKT in renal cell carcinoma. Cancer Research, 2013. PMID: 23824745
Havasi Lab
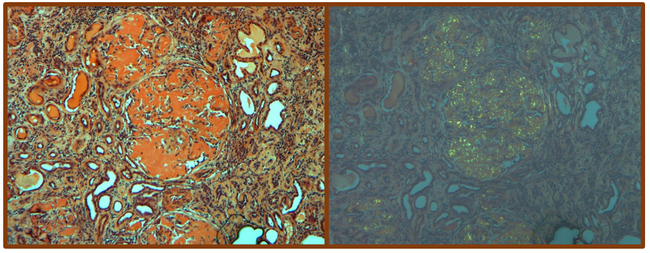
Havasi laboratory conducts basic and translational research in the area of renal injury in amyloidosis and monoclonal immunoglobulin deposition disease. Her weekly amyloidosis clinic has exposed her to the many problems facing amyloidosis patients and created a keen passion to identify and develop new treatment options for amyloidosis patients. She established a productive collaboration with the Amyloid Research Laboratory and, currently, her research focuses on the toxic effects of light chains in the proximal tubule using cell culture and animal models. The main aim is to determine the mechanisms involved in direct kidney cell toxicity focusing on autophagy, the main recycling process in cells. By understanding the mechanism causing kidney cell toxicity we will get closer to finding a therapy which can potentially prevent or cure kidney damage in patients with plasma cell dyscrasia.
Five selected publications:
1. Havasi A et al. Predictive value of the new renal response criteria in AL amyloidosis treated with high dose melphalan and stem cell transplantation. American Journal of Hematology, 2018. PMID: 29430701
2. Prokaeva T et al. Hereditary Renal Amyloidosis Associated with a Novel Apolipoprotein A-II Variant. Kidney International Reports, 2017. PMID: 29270531
3. Menn-Josephy H et al. Renal Interstitial Fibrosis: An Imperfect Predictor of Kidney Disease Progression in Some Patient Cohorts. American Journal of Nephrology, 2016. PMID: 27626625
4. Nolin AC et al. Proteinuria causes dysfunctional autophagy in the proximal tubule. American Journal of Physiology- Renal Physiology, 2016. PMID: 27582098
5. Havasi A et al. Validation of new renal staging system in AL amyloidosis treated with high dose melphalan and stem cell transplantation. American Journal of Hematology, 2016. PMID: 27356490
Ilori Lab
Ilori laboratory focuses on identifying and reducing health disparities in chronic kidney disease and other non-communicable diseases. Our work looks at etiologic factors and modifiers of CKD, with a special emphasis on the effect of diet, biomarkers, and genetics on CKD progression in both low and high-resource countries. We are interested in how precision medicine can be used to reduce health disparities in kidney disease (both within the US and globally).
Five selected publications:
1. Ilori et al. Racial Disparities in Graft and Patient Survival Among Elderly Kidney Transplant Recipients. J Am Geriatr Soc. 2015 Dec; 63(12): 2485–2493. PMCID: PMC4701211
2. Ilori et al. Factors Affecting Willingness to Receive a Kidney Transplant among Minority Patients at an Urban Safety Net Hospital: A Cross-Sectional Survey BMC Nephrology 2015, Nov 21;16(1):191 PMCID: PMC4654893
3. Kaze AD, Ilori et al. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol., 2018 Jun 1;(19)1:125, PMC5984759
4. Ilori et al. Oxidative Balance Score and the Risk of End Stage Renal Disease and Cardiovascular Disease. Am J Nephrol. 2017;45(4):338-345, 2017 Mar 11. PMID: 28285313
5. Ilori et al. Approach to High Volume Enrollment in Clinical Research: Experiences from an All of Us Research Program Site. Clin Transl Sci. 2020;13(4):685-692. PMID: 32004412
Lu Lab
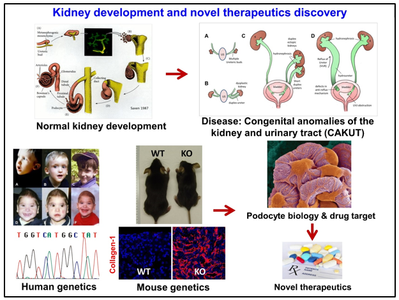
Lu Laboratory focuses on basic science and translational research of kidney development, congenital anomalies of the kidney and urinary tract (CAKUT), podocyte biology and injury, pericyte biology and injury, and discovery of novel drug targets for patient with chronic kidney disease. Lu Lab research program is supported by grants from the National Institutes of Health (NIH) and Pfizer Centers for Therapeutic Innovation.
Five selected publications:
1. Lu W et al. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. American Journal Human Genetics, 2007. PMID 17357069
2. Fan X et al. Inhibitory effects of Robo2 on nephrin: a crosstalk between positive and negative signals regulating podocyte structure. Cell Reports, 2012. PMID: 22840396
3. Hwang DY, Kohl S, Fan X, et al. Mutations of the SLIT2-ROBO2 pathway genes SLIT2 and SRGAP1 confer risk for congenital anomalies of the kidney and urinary Tract. Human Genetics, 2015. PMID: 26026792
4. Rasouly HM et al. Loss of Zeb2 in mesenchyme-derived nephrons causes primary glomerulocystic kidney disease. Kidney International, 2016. PMID: 27591083
5. Fan X et al. SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. Journal of Clinical Investigation, 2016. PMID: 27882344
Rifkin / Bonegio Lab
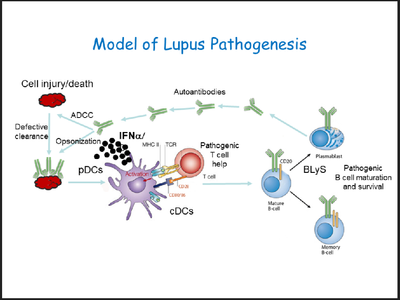
Rifkin / Bonegio Laboratory aims to understand the pathobiology of systemic lupus erythematosus and lupus nephritis with the ultimate goal of identifying new therapeutic targets. Lab uses mouse models and in-vitro-systems as well as human cells and patients samples. Projects in the lab focuses on understanding the mechanisms and signaling pathways for the dendritic cell activation and determining the role of interferon regulatory factor 5 (IRF5) in lupus pathogenesis. In addition, the lab also collaborates with scientists in molecular cardiology and uses a novel mouse model to study the mechanisms of premature atherosclerosis seen in lupus.
Five selected publications:
1. Yasuda K et al. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. Journal of Immunology, 2009. PMID: 19648272
2. Richez C et al. IFN regulatory factor 5 is required for disease development in the FcgammaRIIB-/-Yaa and FcgammaRIIB-/- mouse models of systemic lupus erythematosus. Journal of Immunology, 2010. PMID: 20007534
3. Watkins AA et al. IRF5 deficiency ameliorates lupus but promotes atherosclerosis and metabolic dysfunction in a mouse model of lupus-associated atherosclerosis. Journal of Immunology, 2015. PMID 25595782
4. Aprahamian TR et al. The immunomodulatory parasitic work product ES-62 reduces lupus-associated accelerated atherosclerosis in a mouse model. International Journal of Parasitology. PMID: 25666929
5. Duffau P et al. Promotion of inflammatory arthritis by interferon regulatory factor 5 in a mouse model. Arthritis Rheumatology, 2015. PMID: 26315890
Waikar Lab Waikar Laboratory’s overall goal is to improve the lives of our patients suffering from kidney disease through patient-oriented research. We use a highly collaborative and interdisciplinary approach to perform observational studies, biomarker discovery/validation, clinical trials, and implementation research. Our research projects are purposefully wide ranging and address areas of importance to patient care. Major current projects include the NIH Kidney Precision Medicine Project and research into novel biomarkers and therapeutic targets in AKI and CKD. Other ongoing studies include renal functional reserve; randomized controlled trials to improve the safety of hemodialysis and hemofiltration; optimal diagnostic testing in AKI and CKD; and optimizing care delivery in CKD and AKI.
Waikar Laboratory’s overall goal is to improve the lives of our patients suffering from kidney disease through patient-oriented research. We use a highly collaborative and interdisciplinary approach to perform observational studies, biomarker discovery/validation, clinical trials, and implementation research. Our research projects are purposefully wide ranging and address areas of importance to patient care. Major current projects include the NIH Kidney Precision Medicine Project and research into novel biomarkers and therapeutic targets in AKI and CKD. Other ongoing studies include renal functional reserve; randomized controlled trials to improve the safety of hemodialysis and hemofiltration; optimal diagnostic testing in AKI and CKD; and optimizing care delivery in CKD and AKI.
Five selected publications:
1. Waikar SS et al. Association of urinary oxalate excretion with the risk of chronic kidney disease progression. JAMA Internal Medicine, 2019. PMID: 30830167
2. Leaf D et al. Iron, hepcidin, and death in human AKI. Journal of American Society of Nephrology, 2019. PMID: 30737269
3. Waikar SS et al. Biological variability of estimated GFR and albuminuria in CKD. American Journal of Kidney Disease, 2018. PMID: 30031564
4. Mendu ML et al. The usefulness of diagnostic testing in the initial evaluation of chronic kidney disease. JAMA Internal Medicine, 2015. PMID: 25730699
5. McMahon GM et al. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Internal Medicine, 2013. PMID: 24000014