Graduate Medical Sciences and the Russek Foundation are pleased to award nine high-achieving graduate students and recent alumni with 2024 Henry I. Russek Student Achievement Awards:
The Russek Student Achievement Awards celebrate the ongoing accomplishments of GMS students at Boston University Chobanian & Avedisian School of Medicine. Award winners were chosen not only for their excellent academic research, but also for how they give back to their departments, programs and communities.
The Russek Foundation, led by Professor of Pharmacology Shelley J. Russek, has organized an awards day with a keynote speaker for BU Chobanian & Avedisian SOM and has distributed over $18,000 each year since 1994. The awards honor the memory of Russek’s father, Henry I. Russek, MD, a prolific physician scientist in cardiology, and his wife, philanthropist Elayne Russek. The mission of the RF-endowed program will continue to honor our students but will be repositioned to make the greatest impact for the university in keeping with new areas of need.
2024 marks the final year of the Russek Student Achievement Awards, with plans for GMS to initiate a new research symposium moving forward.
Each award winner received a prize of $1,000. Learn more about our nine awardees below:

Robert Fisher matriculated into the PhD Program in Biomedical Sciences (PiBS) in 2020 and is currently a candidate in the Graduate Program in Genetics & Genomics. He is completing his research in the lab of Professor of Dermatology Rhoda Alani, MD.
Can you briefly describe your research?
I have been studying the interplay between the epigenetic-modifying complex CoREST and RNA splicing mechanisms in melanoma using a synthetic dual warhead inhibitor of CoREST called corin. I found that enzymatically impairing CoREST leads to loss of novel CoREST-splicing factor protein interactions and subsequent RNA splicing changes across an array of melanoma cell lines. These exon skipping events not only reverse oncogenic RNA splicing, but also induce neoantigen production, which could enhance immunogenicity in melanoma.
What inspired you to pursue this field of research?
I would like to think I have always been drawn to genetics as someone who is a triplet. I remember being young and particularly curious about what makes us more or less different from one another. That eventually inspired me to conduct undergraduate research, where I became interested in chromatin organization and how epigenetic mechanisms could dramatically alter gene expression.
Upon entering graduate school, I wanted to expand my basic understanding of chromatin structure to include the translational impact of epigenetic changes in the context of cancer. Interestingly, I did not have much experience with RNA splicing mechanisms, but stumbled upon a connection between the CoREST complex and RNA splicing factors while digging through a proteomics dataset and became enamored with RNA biology and computational methods. That initial finding has not only shaped my research project and graduate studies but has also influenced my career interests.
What are your plans to further explore this research topic?
We plan on testing our findings in vivo for further validation. This will give insight into how this interplay impacts the whole organism.
Back to top

Yi Guan is a rising final-year PhD candidate in the Department of Anatomy & Neurobiology. She previously earned a Master of Science in Anatomy & Neurobiology at BU in 2020. Guan is completing her PhD research in the lab of Bang-Bon Koo, PhD, assistant professor of anatomy & neurobiology.
Can you briefly describe your research?
My doctoral research consists of both wet- and dry- lab techniques, including animal handling, sample collection, tissue analysis, MRI acquisition, imaging processing and computational modeling, using rodent models and human datasets. This wide spectrum of training has been crucial for my dissertation project, where I employ a multi-omics approach to understand the role of the APOE4 allele, a major genetic risk factor for late-onset Alzheimer’s disease (LOAD), during brain and cognitive aging. Building upon insights from animal and human datasets, I will 1) elucidate how genetic predispositions interact with environmental factors to contribute to LOAD pathogenesis, and 2) propose a novel, non-invasive MRI technique for accurate disease prediction and prognosis.
What inspired you to pursue this field of research?
Born in a family of medical doctors, my exposure to science began with bizarre childhood bedtime stories, when my parents enthusiastically talked about laboratory rabbits and lifesaving medications. This early curiosity transformed into a thirst for knowledge as I witnessed my grandfather, once an intelligent schoolteacher and poet, become extremely irritable and could no longer recognize my face. Yet, no treatment could cure his condition. These experiences motivated me to pursue a PhD in Anatomy & Neurobiology at Boston University, where I study the role of the APOE4 allele, a major genetic risk factor for late-onset Alzheimer’s disease, in brain and cognitive aging.
What does receiving the Russek Student Achievement Award mean to you?
Receiving the Russek Student Achievement Award as a member of the Department of Anatomy & Neurobiology will not only be a recognition of my contributions as a young neuroscience researcher, but also strongly encourage me to pursue a career dedicated to tackling the most challenging questions of the human brain. As an international student entering the final year of PhD training, this award will greatly enhance my academic profile and facilitate my search for a postdoctoral role in the US. With a strong commitment to my scientific and professional development, this recognition will motivate me to further excel in a career that bridges gaps in our current understanding and paves the way for innovative treatments for neurodegenerative diseases.
Back to top

Remi Janicot entered BU Chobanian & Avedisian School of Medicine in 2020 through the PiBS program before specializing in the Department of Biochemistry & Cell Biology. Janicot is completing his PhD research under the direction of Mikel Garcia-Marcos, PhD, professor of biochemistry & cell biology.
Can you briefly describe your research?
My research projects revolve around developing new technologies to study G protein-coupled receptors (GPCRs), which are located on the outside of cells and mediate a wide range of physiological processes, including perception of light, neurotransmission, or responses to hormones, among others. GPCRs are also prime drug targets, with over 30% of FDA-approved drugs targeting them and many more compounds in clinical trials. Having appropriate tools to study GPCR signaling is therefore critical for our understanding of cell function in both health and disease.
What research contribution have you made to your field that is most meaningful to you?
Our lab recently published a paper describing the development of a new GPCR biosensor platform with unparalleled capabilities. We have made these sensors accessible for any other lab to use, and my PI and I have already gotten very positive feedback from users. Knowing that the tools we developed are being used and are helping other labs discover new biology is the most exciting and meaningful part of this project.
What does receiving the Russek Student Achievement Award mean to you?
It is a tremendous honor that I do not take for granted. It is a testament to the quality of the research that goes on not only in the Garcia-Marcos lab, but in the entire Biochemistry & Cell Biology Department.
Back to top

Daniel Kirsch is an MD/PhD candidate at BU Chobanian & Avedisian School of Medicine, where he matriculated in 2016. Kirsch is completing his PhD research at the Chronic Traumatic Encephalopathy (CTE) Center under the direction of William Fairfield Warren Distinguished Professor Ann McKee, MD.
Can you briefly describe your research?
CTE is a neurodegenerative disease associated with exposure to repetitive head impacts (RHI) and is characterized by accumulation of hyperphosphorylated tau protein (p-tau). The p-tau pathology of CTE is enhanced by post-traumatic neuroinflammation and microvascular damage. My research was focused on establishing the role of neurovascular inflammation and the presence of substantia nigra pathology within CTE pathogenesis, and how neuropathological findings correlated with clinical symptoms, especially parkinsonism.
We found that years of exposure to head impacts corresponded to the measures of neurovascular inflammation, which were in turn associated with increased measures of microgliosis and p-tau pathology. We found increased rates of parkinsonism in CTE and that parkinsonism was related to RHI exposure duration, nigral neuron loss and p-tau pathology. Finally, we found that CTE p-tau pathology consistently impacted subcortical nuclei, including the substantia nigra, and that p-tau pathology and neuron loss in those regions were associated with clinical and functional impairment.
What inspired you to pursue this field of research?
I have been interested in pathology since beginning medical school, which made working at the VA-BU-CLF brain bank under Dr. McKee a perfect fit. I was highly interested in learning about CTE and the drivers of the pathology we see histologically. This research allowed me a unique opportunity to work with human samples and correlate analytical findings with clinical symptoms.
What research contribution have you made to your field that is most meaningful to you?
My work concerning CTE and parkinsonism [is most meaningful to me]. This symptom had been noted before, but it was not well understood what the drivers of clinical symptoms were. By doing this research, we are able to highlight motor symptoms as a component of CTE and yet another risk associated with exposure to repetitive head impacts. Hopefully, the recognition of drivers of these devastating symptoms will allow for future work to develop treatments.
Back to top
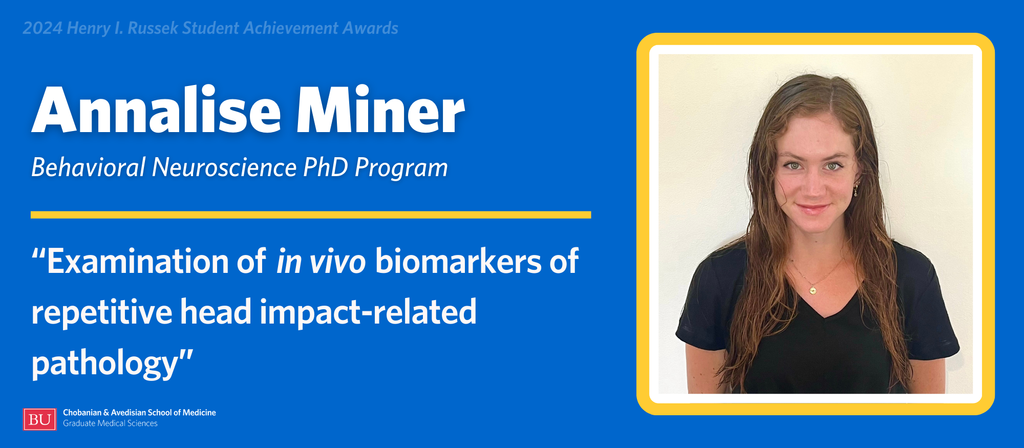
Annalise Miner is a candidate in the Behavioral Neuroscience PhD Program. She is completing her research under the direction of Associate Professor of Neurology Michael Alosco, PhD.
Can you briefly describe your research?
My research is centered around examining in vivo biomarkers of repetitive head impact exposure and chronic traumatic encephalopathy. I am working on examining different kinds of plasma, cerebrospinal fluid and neuroimaging biomarkers to help better our understanding of how pathologies related to repetitive head impacts develop and lead to cognitive changes.
What inspired you to pursue this field of research?
I was inspired to pursue research in the field of CTE due to the number of people that are affected by the disease and how we are unable to diagnose it during life. I was interested in how we can leverage biomarkers to understand how a disease starts, progresses and most importantly, how we can treat it.
What research contribution have you made to your field that is most meaningful to you?
The contribution that I have made to my field that is most meaningful to me is our study examining a panel of plasma biomarkers in a cohort of former football players. We found moderate correlations of these plasma biomarkers with metrics of repetitive head impact exposure, but it was one of the first studies examining these biomarkers in a large cohort of participants. This was meaningful to me because it was my first contribution to the field of CTE-related research and was a unique and fulfilling opportunity to grow as a researcher.
Back to top
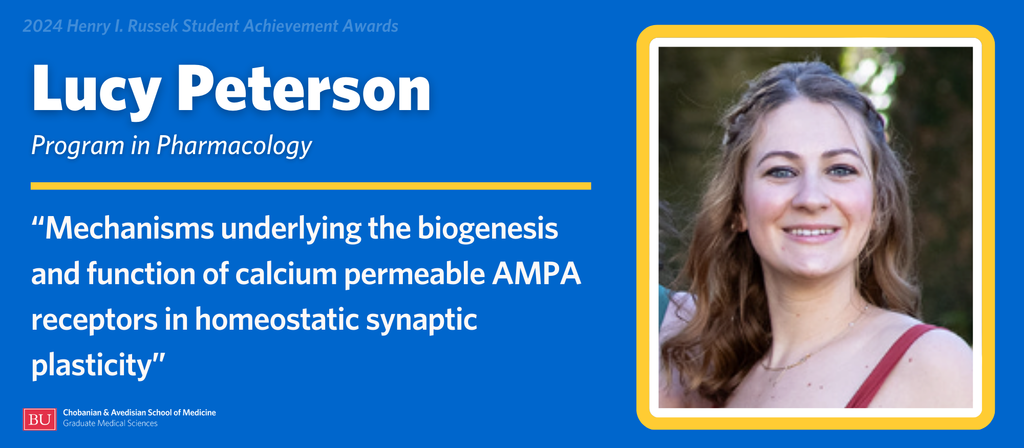 Lucy Peterson is a candidate in the PhD Program in Pharmacology. She is completing her dissertation research under the direction of Professor of Biology Hengye Man, MD, PhD.
Lucy Peterson is a candidate in the PhD Program in Pharmacology. She is completing her dissertation research under the direction of Professor of Biology Hengye Man, MD, PhD.
Can you briefly describe your research?
Neurons rely on homeostatic mechanisms to maintain network stability after perturbation by stimuli. One important mechanism to maintain homeostasis is homeostatic synaptic plasticity (HSP). To induce HSP after activity deprivation, calcium permeable AMPA receptors (Cp-AMPARs) are rapidly inserted into the synapse. This drives AMPA receptor synaptic accumulation, thus restoring neuronal firing rates. However, it is still not clear what mechanisms underly the formation of Cp-AMPARs and the role of calcium in triggering synaptic accumulation of AMPA receptors. In my project, I’ve identified that alternative mRNA editing of the AMPA receptor subunit GluA2 is crucial for the formation of Cp-AMPARs and the induction of HSP. Separately, I have found that the increase in Cp-AMPAR-mediated calcium leads to increased GluA1 acetylation and synaptic retention, which is crucial for HSP expression.
What inspired you to pursue this field of research?
I have always had an interest in molecular biology and felt inspired to integrate molecular biology with neuroscience. I especially am passionate about studying the mechanisms underlying learning and memory, as these are the processes that make humans complex, advanced and individual. I believe that understanding these mechanisms will greatly further our knowledge of human behavior and cognition. I am grateful to have been able to pursue this study with Dr. Man, who is a leader in the field of neuronal plasticity and a fantastic mentor.
What does receiving the Russek Student Achievement Award mean to you?
Receiving the Russek Award is a great privilege, and I am appreciative to have chosen by the Pharmacology department. I have had an amazing experience at BU, and I am grateful for all of the opportunities provided to me by the program and all of the wonderful people in the department. I have been eager to contribute to the success of the department in any way that I could and am so honored to be representing Pharm as the Russek Award winner!
Back to top
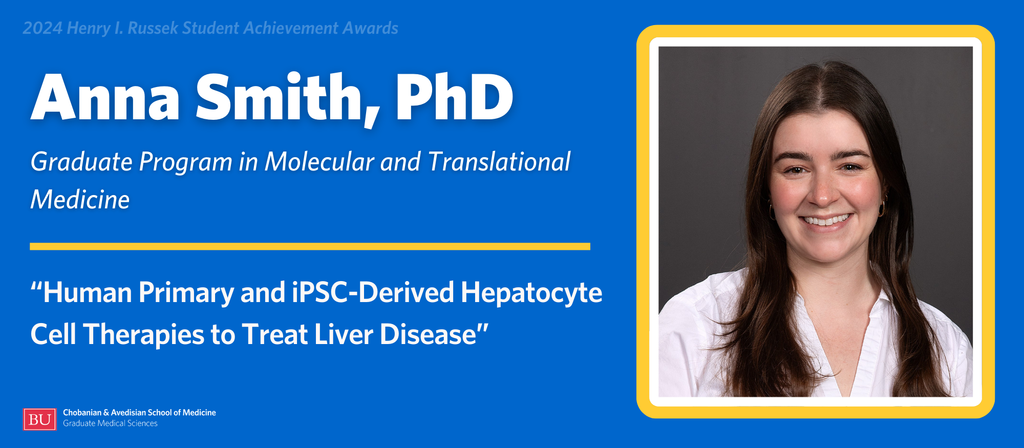
Anna Smith, PhD, is a May 2024 graduate of the Graduate Program in Molecular and Translational Medicine. She completed her dissertation research in the lab of Associate Professor of Medicine Valerie Gouon-Evans, PhD, PharmD, and successfully defended in March 2024. Smith is also a 2024 recipient of the GMS Outstanding Student Achievement Award in PhD Research.
Can you briefly describe your research?
Currently, the main treatment for end stage liver disease is a liver transplantation; however, there is always a donor organ shortage. My research project is focused on finding alternatives to liver transplantation. What we propose is to treat liver diseases by transplanting healthy liver cells instead of an entire organ. I have been exploring liver cell transplantation in mouse models that mimic actual human liver diseases. We discovered in the lab that liver cell transplantation alone is not sufficient to treat liver disease. Next, we started administering supportive factors to help transplanted cells.
Excitingly, we found that this significantly improved liver cell engraftment and actually helped treat liver disease in mice. To deliver these supportive factors to transplanted cells, we took advantage of the nucleoside modified mRNA in lipid nanoparticles (mRNA-LNP) platform. The mRNA-LNP platform is the same technology that is used for Covid19 vaccines, which means that we are developing a therapeutic that has strong potential to be used in humans too. This is the project that lead to my recognition from the Russek Foundation. We also just published all of this work so that it is available to the greater scientific community!
What research contribution have you made to your field that is most meaningful to you?
My research has the potential to overcome critical barriers to the clinical translation of liver cell therapies. Currently, liver cell therapies are not widely used to treat human patients. But with discoveries that I made in the lab, along with the help of all of my colleagues, liver cell therapies are a more viable alternative to organ transplantation than ever before. This research has the potential to benefit many patients suffering from liver disease.
What are your plans to further explore this research topic?
I am looking forward to further exploring liver related therapeutics by working at a biotech company after graduation.
Back to top

Erika Smith-Mahoney matriculated into BU Chobanian & Avedisian School of Medicine through the PiBS program in 2020 before specializing in the Department of Virology, Immunology & Microbiology. She is completing her dissertation research in the lab of Jennifer Snyder-Cappione, PhD, assistant professor of virology, immunology & microbiology.
Can you briefly describe your research?
My research focuses on investigating alterations of gamma delta T cells during chronic inflammatory states, such as ART-suppressed HIV infection and aging. I am determining the phenotypic and functional signatures of circulating gamma delta T cells using fluorescence and mass cytometry, respectively. Also, using imaging mass cytometry, we are visualizing tissue resident gamma delta T cells in the gastrointestinal system. These combination approaches enable integrative and comparative analyses of the complexities and role of blood and gut gamma delta T cells in chronic inflammation. Ongoing work indicates the existence of both pro- and anti-inflammatory factions of gamma delta T cells which may influence aberrant inflammation and contribute to perturbations in intestinal barrier integrity.
What research contribution have you made to your field that is most meaningful to you?
My most meaningful contribution to the field of gamma delta T cell research is our most recent publication in the Journal of Infectious Diseases, “Vd1 Effector and Vd2 gd T-Cell Subsets Shift in Frequency and Are Linked to Plasma Inflammatory Markers During Antiretroviral Therapy-Suppressed HIV Infection”, published in May 2024 and presented at the International Gamma Delta T Cell Conference (Lisbon, Portugal 2023). Using high-dimensional flow cytometry on peripheral blood mononuclear cells from individuals in our HIV and Aging cohort, we identified 39 distinct gamma delta T cells subsets which were differentially and significantly altered in ART-suppressed HIV infection and demonstrated either innate- or adaptive-like features. This research also suggested selective links of plasma inflammatory markers with the frequencies of multiple subsets of gamma delta T cells, further providing evidence that distinct subsets can exert either pro- or anti-inflammatory functions in chronic inflammatory conditions.
What does receiving the Russek Student Achievement Award mean to you?
It is an incredible honor to be selected as the recipient of the 2024 Russek Student Achievement Award for the Department of Virology, Immunology, and Microbiology. All students, not only within the VIM department, but Graduate Medical Sciences and the PiBS program, make significant contributions to science in their respective fields and persevere through the highs and lows of graduate school programs. I am humbled to be awarded this honor, as there are many students that have demonstrated rigor in their research, continue to effectively communicate science in the age of misinformation in the media and have mentored many throughout their PhD journey. I am proud to represent all my peers in receiving this award as we work together to propel science, continue to make discoveries, and inspire inclusivity, diversity of minds, and excellence.
Back to top

Kelton Wilmerding is a January 2024 graduate of the Graduate Program for Neuroscience. He completed his dissertation research in the lab of Professor of Psychological & Brain Sciences Michael Hasselmo, director of the BU Center for Systems Neuroscience. Wilmerding successfully defended his dissertation in November 2023.
Can you briefly describe your research?
Activity-dependent tagging of neural ensembles allows visualization and manipulation of memory-related neurons. To determine the impact of artificial memory ensemble activation on neural dynamics, we tagged and artificially stimulated a group of cells in the hippocampus while recording electrophysiology from multiple brain regions. Our current results indicate that artificial stimulation entrains local circuit dynamics in the stimulated region and in downstream areas, while acutely and predictably modifying the neural code for space in the hippocampus.
What inspired you to pursue this field of research?
I was drawn to the field of learning and memory in neuroscience because memories are one of the most enriching and meaningful components of our mental world. Contributing to our understanding of how the brain forms, stores and retrieves memory is a huge passion of mine. We even draw on memory when we imagine the future or create art. And on the other hand, when memories fail us in old age or disease, it can be heartbreaking to see those we love slip away into dementia.
What does receiving the Russek Student Achievement Award mean to you?
Receiving the Russek Student Achievement Award reminds me of all the colleagues, mentors and friends who I’ve had with me throughout my ongoing scientific journey. I’m truly honored to think about the time, dedication and trust placed in me by the many teachers who helped me get where I am today. Not to mention, I’m hugely grateful to my peers who were with me in the trenches when experiments failed or troubleshooting needed to happen. The award reminds me of how far I’ve come, who got me here and where I’m going next.
Back to top
View all posts

 Lucy Peterson is a candidate in the PhD Program in Pharmacology. She is completing her dissertation research under the direction of Professor of Biology Hengye Man, MD, PhD.
Lucy Peterson is a candidate in the PhD Program in Pharmacology. She is completing her dissertation research under the direction of Professor of Biology Hengye Man, MD, PhD.