Research Week 2022 – Kevin Stirling, MD
Learning Points:
- Describe a case of cytokine release syndrome
- Review the management of cytokine release syndrome
- Review the literature about immunotherapy, mRNA vaccinations, and cytokine release syndrome.
Case Title: Cytokine Release Syndrome after combination of Immunotherapy and mRNA Vaccination.
Case History:
68 F presented to primary care with 2 lumps in R breast. Mammogram was performed with highly suspicious R breast mass and abnormally enlarged axillary and intramammary lymph nodes, BI-RADS 5. Core biopsy was performed with pathology revealing invasive ductal carcinoma, grade III, ER negative, PR negative, Her2neu negative. Sample lymph node was positive for metastatic carcinoma. She was seen by surgical and medical oncology. Staging scans including CT chest, abdomen and pelvis, and NM bone scan demonstrated extensive disease in breast, axillary and supraclavicular lymph nodes, but did not reveal definite metastatic disease (a 1.2 cm hilar node was interpreted as equivocal for metastatic disease). Plan was made to treat with chemo/immunotherapy based on Keynote 522 results. She started on treatment with pembrolizumab, carboplatin, paclitaxel.
Patient presented to oncology clinic for C2D15. At that visit she noted body aches, bilateral knee pain and lightheadedness (fell one week prior to visit). Also complained of fatigue and intermittent numbness in fingers for previous three days. Her vital signs were T: 97.6, HR: 85, BP: 137/75, RR: 18, SpO2: 100%. Her documented physical exam was within normal limits, including normal mood, affect, behavior, judgment and thought content. Her ECOG performance status was 1. Her blood work included WBC: 5.4, Hgb: 11.3, HCT: 35.4, PLT: 200, Na: 139 K: 3.6, Cl: 104, CO2: 24, BUN: 9, Cr: 0.71, Glu: 151, Tbili: 0.3, AST: 15, ALT: 20, ALP: 55. She proceeded with infusion of pembrolizumab, carboplatin, paclitaxel. She was additionally given a third booster shot of the Pfizer Covid 19 vaccine.
The next day, C2D16, patient was brought by EMS to the emergency department for altered mental status after being found down on the floor at home. On arrival patient not responding coherently to any questions. Last known well was the evening prior around 6 pm, at which time patient was complaining of a headache over the phone to daughter. Initial vital signs in the emergency department were T: 104.7, HR: 127, BP: 136/58, SpO2: 76%. Exam documented as patient opening eyes to her name but not following commands, tachycardic and tachypneic, with dry mucous membranes. Labs with WBC 7.2, Hgb: 13.8, Hct: 43, Plt: 163, INR: 1.43, Na: 139, K: 4.8, Cl: 105, CO2: 16, BUN: 21, Cr: 2.30, Glu: 111, AST: 771, ALT: 222, ALP: 141, Troponin: 2.528, Lactate: 5.1, procalcitonin 41.16, CK: 23,342, BNP: 243, LDH: 1841, VBG: 7.32/43. A comprehensive respiratory panel including Covid 19 was negative. Urinalysis with 1+ protein, 2+ blood, neg leukocyte esterase, negative bacteria, negative WBC. Chest x ray, CT head, CT pulmonary angiogram and CT abdomen/pelvis were all obtained and largely unremarkable (no evidence of splenomegaly). Blood cultures were drawn, IV Fluids, ceftriaxone, vancomycin and acetaminophen suppository given, and patient admitted to MICU. After arrival to the MICU patient was intubated for airway protection/hypoxemia and started on pressor support with norepinephrine. Lumbar puncture was attempted but not successful. Antibiotics were broadened to include empiric meningitis coverage.
Initial differential diagnosis included septic shock, cytokine release syndrome, and HLH. No clear infectious source was noted on workup. Blood cultures, urine culture, LP (obtained twice with neuro interventional team), lower respiratory tract panel, fungal workup (aspergillus, DFA) all eventually came back unremarkable. Ferritin was significantly elevated (>33,000) with normal fibrinogen (307), Triglycerides 587. IL-6 level was 108.9 and soluble IL-2 receptor 3699.8. In consultation with oncology team decision was made to start methylprednisolone 1g daily on HD 2 to treat for cytokine release syndrome (grade 4 as patient had been intubated). This decision was guided by previous case reports. On HD 3 patient began having clinical seizures with EEG showing ictal phenomenon. Decision was then made to give tocilizumab 8mg/kg. MRI/MRA was performed with no significant findings. Empiric antibiotics were stopped with negative workup as noted. Methylprednisolone was subsequently tapered then transitioned to dexamethasone and stopped on HD 17.
Cr continued to rise and urine output dropped over the course of hospital stay until HD 5 when patient was noted to have only 110 mL UOP in 24 hours (and net positive 10 L at that point), with Cr up to 4.8 – dialysis catheter was placed and she was started on CVVH. She subsequently had intermittent HD until her kidney function showed slow improvement and dialysis catheter was removed on HD 41.
Patient did resume cancer treatment while hospitalized; she was started on paclitaxel therapy on HD 26. She was discharged on HD 56 to rehab. Since discharge she has continued on single agent paclitaxel. Her subsequent course has been complicated by recurrent c.diff infections, and as of her last clinic note she remains wheelchair bound and in rehab, ~6 months after initial presentation.
Discussion
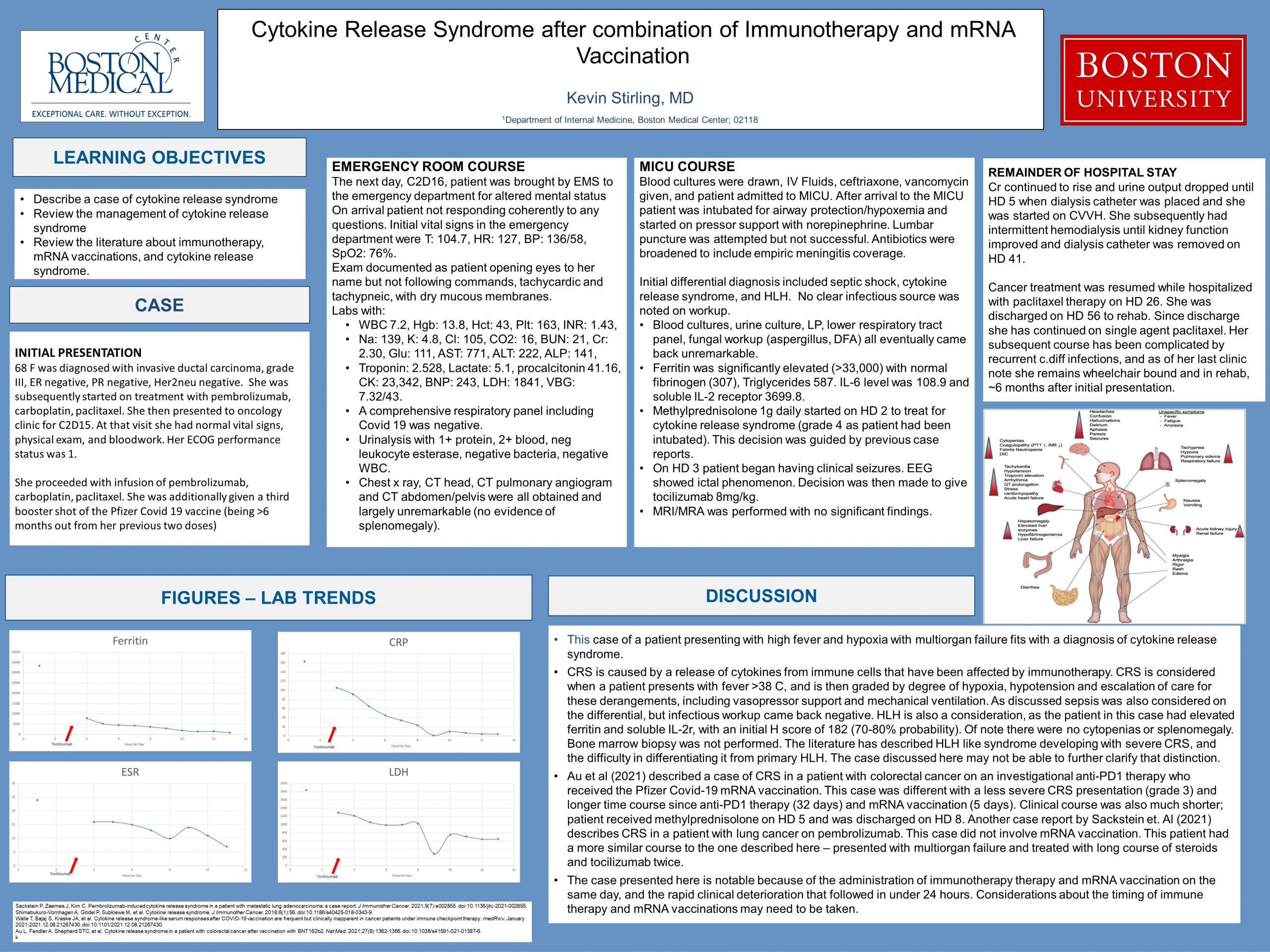
This case of a patient presenting with high fever and hypoxia with multiorgan failure fits with a diagnosis of cytokine release syndrome. CRS is caused by a release of cytokines from immune cells that have been affected by immunotherapy. CRS is considered when a patient presents with fever >38 C, and is then graded by degree of hypoxia, hypotension and escalation of care for these derangements, including vasopressor support and mechanical ventilation. Cases can vary from mild to multiorgan failure. As discussed above sepsis was also considered on the differential, but infectious workup came back negative. HLH is also a consideration, as the patient in this case had elevated ferritin and soluble IL-2r, with an initial H score of 182 (70-80% probability). Of note there were no cytopenias or splenomegaly. Bone marrow biopsy was not performed. The literature has described HLH like syndrome developing with severe CRS, and the difficulty in differentiating it from primary HLH. The case discussed here may not be able to further clarify that distinction.
The temporal link to immunotherapy and mRNA vaccination suggests this combination as an etiology for the CRS. CRS in association with immunotherapy and mRNA vaccination is rare, however cases have been described in the literature. Au et al (2021) described a case of CRS in a patient with colorectal cancer on an investigational anti-PD1 therapy who received the Pfizer Covid-19 mRNA vaccination. This case was different with a less severe CRS presentation (grade 3) and longer time course since anti-PD1 therapy (32 days) and mRNA vaccination (5 days). Clinical course was also much shorter; patient received methylprednisolone on HD 5 and was discharged on HD 8. Another case report by Sackstein et. Al (2021) describes CRS in a patient with lung cancer on pembrolizumab. This case did not involve mRNA vaccination. This patient had a more similar course to the one described here – presented with multiorgan failure and treated with long course of steroids and tocilizumab twice. This treatment was similarly employed in the case described here, and supports it as a management strategy.
The case presented here is notable because of the administration of immunotherapy therapy and mRNA vaccination on the same day, and the rapid clinical deterioration that followed in under 24 hours. This will be an area of future interest, as patients with malignancy are considered high risk groups for infections, and it is possible that mRNA vaccinations will expand in the future to cover more infectious diseases. Considerations about the timing of immune therapy and mRNA vaccinations may need to be taken. Some researchers have already taken up this question – Walle et al. (2021)l have a pre-print clinical trial article out about monitoring for cytokine release syndrome like responses in patients on checkpoint inhibitors receiving covid vaccinations. They compared serum markers in vaccinated versus unvaccinated patients, with their discussion summarizing that while CRS markers were more elevated in vaccinated patients they were clinically inapparent.
References:
- Sackstein P, Zaemes J, Kim C. Pembrolizumab-induced cytokine release syndrome in a patient with metastatic lung adenocarcinoma: a case report. J Immunother Cancer. 2021;9(7):e002855. doi:10.1136/jitc-2021-002855.
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi:10.1186/s40425-018-0343-9.
- Walle T, Bajaj S, Kraske JA, et al. Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent but clinically inapparent in cancer patients under immune checkpoint therapy. medRxiv. January 2021:2021.12.08.21267430. doi:10.1101/2021.12.08.21267430.
- Au L, Fendler A, Shepherd STC, et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med. 2021;27(8):1362-1366. doi:10.1038/s41591-021-01387-6.