Research Week 2021 – Brett Dumas, MD
Brett Dumas, MD
Clinical Research
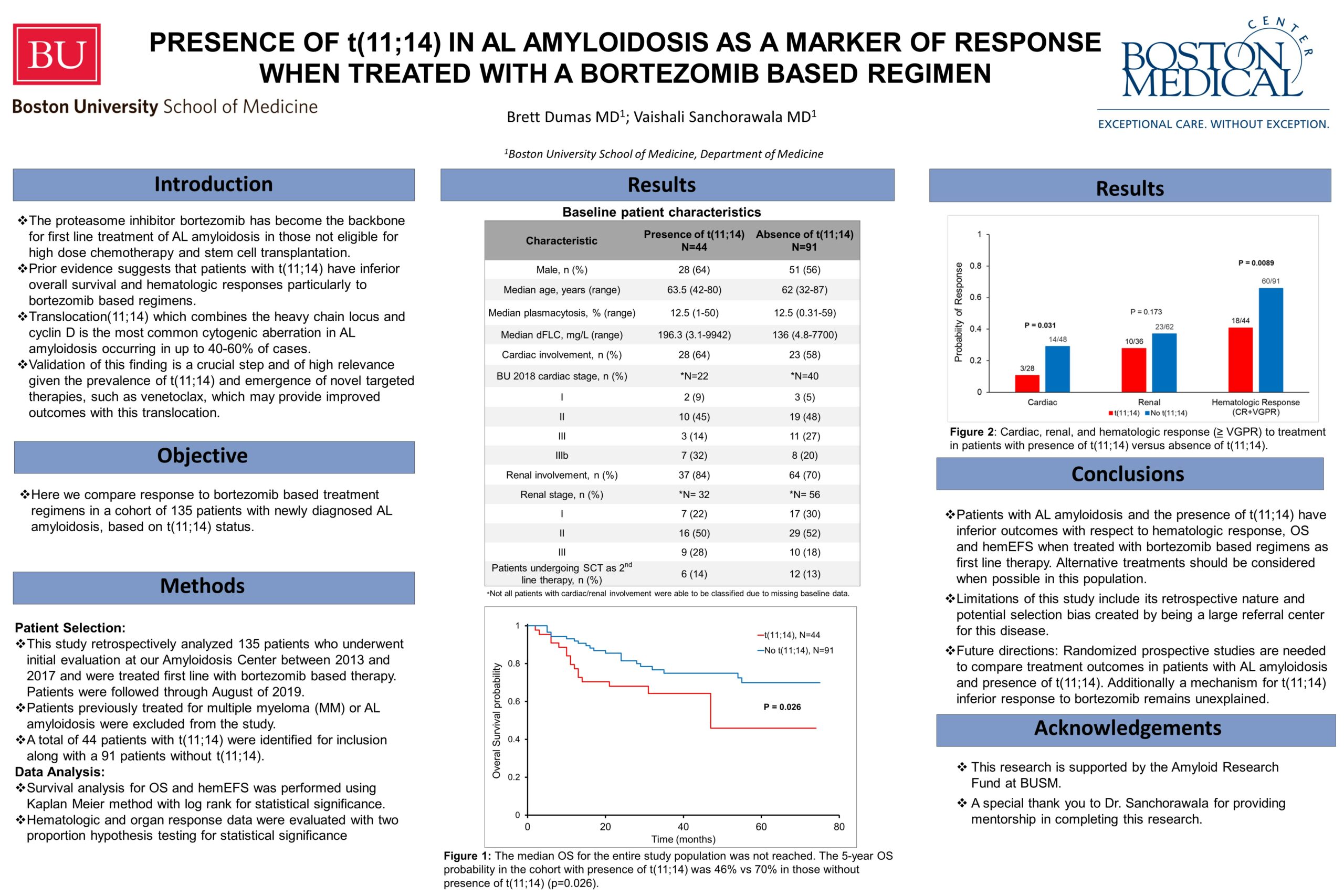
The proteasome inhibitor, bortezomib, has become a backbone for the first line treatment of patients with AL amyloidosis who are not eligible for high dose melphalan and stem cell transplantation. The presence of t(11;14), seen in up to 40-60% of patients with AL amyloidosis, may be associated with poorer response when treated with bortezomib based regimens. This remains a critical distinction in light of recent evidence demonstrating favorable responses to BCL-2 inhibition with venetoclax in patients with t(11;14) in multiple myeloma. We report on 135 patients with newly diagnosed AL amyloidosis treated with a bortezomib-based regimen as first line therapy between 2013 and 2017. Treatment outcomes were compared between a cohort of patients with t(11;14) and those without the translocation. Of 135 eligible patients, forty-four patients had the presence of t(11;14). Five-year overall survival was 46% for those with t(11;14) and 72% in patients without this translocation (P=0.026). The median hematologic event free survival was 17 months for patients with t(11;14) compared to 34 months without (p=0.068). Best hematologic response (CR+VGPR) was achieved in 41% of patients with t(11;14) vs 66% without t(11;14) (p=0.012). Cardiac and renal responses to first line treatment with bortezomib-based regimens were also higher in patients without t(11;14). In conclusion, patients with AL amyloidosis and the presence of t(11;14) have inferior outcomes with respect to survival, as well as hematologic and organ responses, when treated with bortezomib-based regimens as first line therapy.