Human Subjects Protection Training Certificates
Two types of certification are required for anyone who wants to do human subjects research at Boston University Chobanian & Avedisian School of Medicine:
- A certificate of completion of Human Subjects’ Protection Training from Collaborative Institutional Training Initiative (CITI)
- A certificate of completion of the HIPAA Module
Each prospective researcher must have certificates for both Human Subjects Protection Training and the HIPAA Module on file in the Instiutional Review Board (IRB) office before she/he can begin participation in a research protocol involving human subjects. This includes any research activities such as: assessment of medical records collected as part of routine medical care at Boston Medical Center for prospective or retrospective research studies, quality initiatives, questionnaires and consent of research subjects to participate in prospective research projects.
Human Subjects’ Protection Training
How to obtain your initial Human Subject Research Certification and gain access to BU IRB electronic system (INSPIR II):
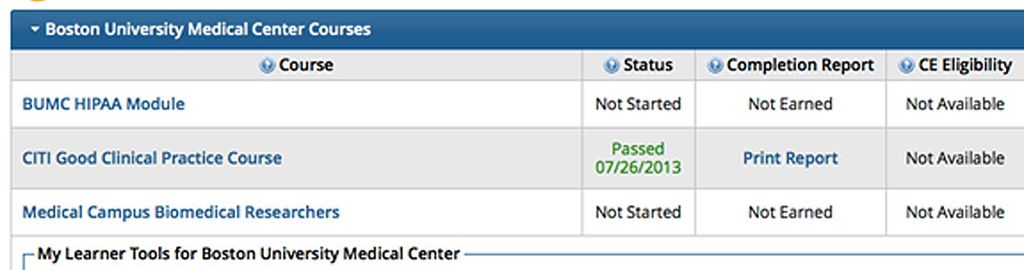
- Initial Human Subjects’ Protection Training must be done through CITI: https://www.citiprogram.org/
- Detailed instruction on how to register for a CITI course can be found at: http://www.bumc.bu.edu/ocr/instructions-for-taking-bumc-citi-courses/
During registration, be sure to select “Boston University Medical Center” as your institutional affiliation (do not pick the “Boston University” option). Also, be sure to list your “@bu.edu” email address as the preferred email address. After entering other information about yourself, you will be presented with several curriculum options. As part of initial certification, you should select the curriculum for “Medical Campus Biomedical Researchers“.

Please Note: BU IRB no longer offers on-site training for human subjects’ protection training certification and does not accept the NIH/NCI on-line training to meet the requirements of Human Subjects’ Protection Training.
HIPAA Module Certification
For HIPAA Module Certification go to the CITI website: https://www.citiprogram.org/. From the list of available courses (see below), select “BUMC HIPAA Module”. When you have completed the module, CITI automatically sends a notice of completion to the BU IRB office.
Once you have completed both training requirements, please provide an electronic copy of your certifications to: Marina Malikova, PhD, MA, CCRA, Director of Surgical Translational Research, Operations and Compliance.
Email: mmalikov@bu.edu
Phone: 617.414.6836
Fax: 617.414.6835