Hans Dooms
Adjunct Assistant Professor of Medicine and Virology, Immunology & Microbiology Boston University School of Medicine Assistant Professor, National Jewish Health
Research Interest
The broad goal of my research is to understand the role of T cells in the pathogenesis of autoimmune diseases and to apply this knowledge for the development of new therapeutic interventions. My laboratory uses in vivo rodent models of Type 1 Diabetes (T1D) and Scleroderma (Systemic Sclerosis, (SSc)) to follow T cell responses against tissues (e.g. pancreatic islets, skin) and to identify molecules (e.g. cytokines) that orchestrate the autoimmune attack. In addition, we use clinical samples from T1D and SSc patients to corroborate the relevance of our findings for human disease.
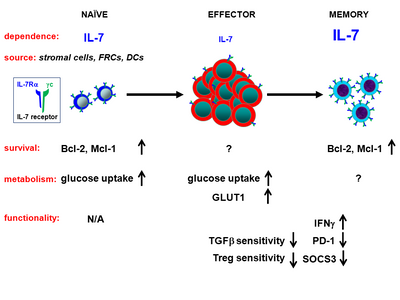
To combine the capacity for potent protective responses against pathogens with the prevention of autoimmunity and tissue damage, the immune system works with “checks and balances” to generate the appropriate cellular response to foreign antigens while maintaining tolerance to self. Autoimmune diseases result from breakdowns of these control mechanisms, either due to defects in tolerance-inducing pathways or because autoreactive lymphocytes acquire resistance to proper regulation. I am particularly interested in the contribution of one type of lymphocytes, memory T cells, to the autoimmune process. Memory T cells possess superior effector capacity and long-term viability to fulfill their physiologic function of protecting the host against recurring infections and tumors. However, memory T cells that develop against self-antigens are, precisely due to these characteristics, a significant clinical problem and a major obstacle to restoring tolerance for the therapy of autoimmune diseases and the protection of transplants.
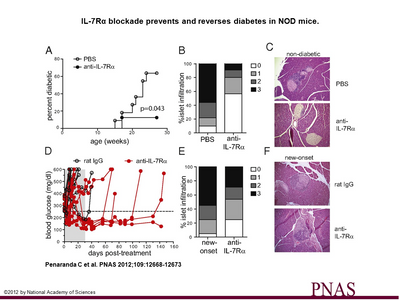
We are currently focusing our effort on understanding regulation of memory T cells in two autoimmune diseases, T1D and SSc. In T1D, we discovered that blocking the cytokine Interleukin-7 (IL-7) inhibits pathogenic memory cells and stops further destruction of the insulin-producing cells in the pancreas. The goals of ongoing projects in the lab are to (1) combine IL-7R blockade with other therapeutic approaches to accomplish robust prevention and reversal of T1D, (2) elucidate the molecular and cellular mechanisms underlying IL-7’s role in autoimmune diseases and (3) determine the role of regulatory and memory T cells in the etiology of sclerodermatous GvHD and human SSc.