Researchers Capture Images of Open Channel That Moves Proteins Across Cell Membranes
(This video shows a bacterial ribosome docked onto the SecYEG protein translocation channel, then removes the ribosome as it zooms in to show the channel as a set of ribbons that represent alpha helices of the 3 different channel subunits. The SecY channel is viewed from the plane of the membrane and changes from a closed to an open form and then the nascent (newly forming) protein (in green) from the ribosome, which is not shown in later stages of the video, is modeled within the central pore of the open channel in a looped configuration and additionally, there is a loop of the new protein lying on the top of the channel, before it enters the central pore.)
Similar to passengers on an urban transit system, every protein made in the cell has a specific destination and function. Channels in cell membranes help direct these proteins to their appropriate target. Researchers at Boston University School of Medicine (BUSM) and their colleagues have now captured images of these channels as they open to allow proteins to pass through a membrane, while the proteins are being made. These findings are published as a Letter in Nature (Park, E. et al. 2013).
Christopher W. Akey, PhD, professor of physiology and biophysics at BUSM is a co-senior author of the Letter. In addition, the collaborating institutions include Harvard Medical School (HMS), Baylor College of Medicine (BCM) and Georgia Institute of Technology (GT).
Proteins, which are encoded by genes, are large molecules that perform specific functions. Many proteins such as hormones and growth factors are secreted by the cell and move into the bloodstream. These proteins are made in factories called ribosomes, which interact with a family of channels called Sec61/SecY that provide a path across the membrane. Initially, these nascent, or newly-made, proteins are inserted into channels as the proteins are being made. The channels also aid in inserting nascent proteins into the cell membrane where they function as receptors for drugs and form ion channels that function in vision and in transmitting nerve cell impulses.
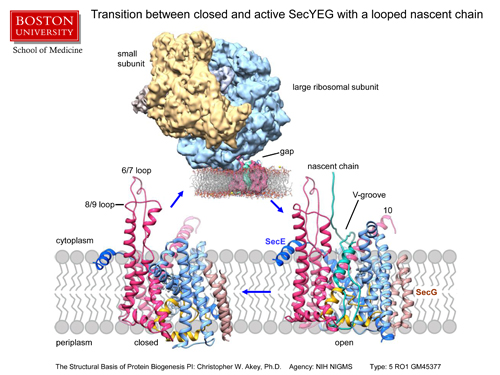
This is a summary slide that shows closed and open ribbon models of the SecYEG channel aligned on a cartoon of the membrane and the open form of the channel on the right has a nascent protein in green that is based on our modeling with the density map. The map itself of the open conformation of the channel and ribosome is shown above, as viewed again along the plane of the membrane, wherein the membrane has been modeled with a computer. The small subunit of the ribosome is in gold, the large subunit in blue, the new protein density is shown in green and the segmented channel density is shown in maroon. The original membrane density in the map has been replaced with a model of a membrane generated by a computer for clarity and is rendered so one can see into the lateral gate of the open channel, ie the green density of the newly made protein is visible.
In this study, researchers used samples made in E. coli bacteria to determine the structure of the highly conserved SecY channel. Using an electron microscope and computer analysis, researchers were able to capture images of the SecY channel opening when a nascent protein enters the central pore. In particular, the channel undergoes large movements that enlarge the central pore as a first step in allowing the nascent protein to cross the cell membrane and eventually travel to its destination.
“Similar to train cars that transport passengers through a tunnel, SecY/Sec61 channels help nascent proteins move across the cell membrane to reach their target in the body, and this study provides important insight about the function of these channels,” said Akey.
Funding for this study was provided in part by the National Institutes of Health’s National Institute of General Medical Sciences under grant award number NIH GM45377.
Tom Rapoport, PhD, professor of cell biology, HMS, is a co-senior author. Other collaborators include: Eun Yong Park, PhD, HMS; Jean-François Ménétret, PhD, BUSM; Steven J. Ludtke, PhD, BCM; and JC Gumbart, PhD, GT.
View all posts