Gustavo Mostoslavsky, M.D., Ph.D.

Professor of Medicine and Virology, Immunology & Microbiology
Co Director at Center for Regenerative Medicine (CReM)
670 Albany Street; Room 209
617-358-1018
gmostosl@bu.edu
M.D. from the University of Tucuman in Argentina
Ph.D. from the Hebrew University in Jerusalem, Israel
See BU Profile for additional information and publications.
Mostoslavsky Lab Website
Embryonic Stem Cell Modeling of Intestinal Differentiation
Embryonic Stem Cells (ESC) are pluripotent undifferentiated cells capable of giving rise to cells from all three germ layers. This unique ability makes them ideal candidates to model early development allowing us to study the basic signaling mechanisms involved in stem cell fate determination. At the same time, manipulating ESC differentiation toward a specific developmental pathway holds a great promise for their use in regenerative medicine. One focus of our lab is differentiating mouse ESC into intestinal epithelial cells in order to understand the complex signaling pathways involved in intestinal commitment from endodermal progenitors and undifferentiated stem cells.
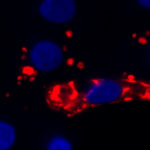
iPS cells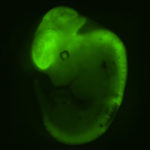
Our lab has a major interest in the study of induced Pluripotent Stem cells or iPS cells and the development of tools for their generation and characterization. Pioneering work by the laboratory of Dr. Yamanaka showed that fibroblasts transduced with retroviral vectors expressing four transcription factors, Oct4, Klf4, Sox2 and cMyc can be reprogrammed to become pluripotent stem cells that appear almost indistinguishable from ESC. In contrast to ESC, iPS cells are genetically identical to the individual from whom they are derived, raising the prospect of utilizing iPS cells for autologous cell based therapies without risk of rejection. We have previously developed a single lentiviral vector expressing a stem cell cassett, named STEMCCA, capable of generating iPS cells from post-natal fibroblasts and peripheral blood with very high efficiency that became the industry standard. We have also modified it to make it excisable and have used it to generate mouse and human iPS cells free of exogenous transgenes. We now aim at using iPS cells for disease modeling disease modeling and study their potential for regenerative medicine.
IPS Cell Modeling of Intestinal Differentiation
One major focus of the lab is the utilization of iPS Cells for the study of intestinal differentiation with a particular interest in Colorectal Carcinoma (CRC). For this purpose we have generated iPSC from individuals suffering from FAP and Lynch Syndrome, the two most highly penetrant hereditary forms of CRC. We have established in the lab robust differentiation protocols for the induction of intestinal specification of these disease-specific iPSC. By studying the earlier events associated with intestinal differentiation comparing the normal and mutant cells we aim at discovering the basic mechanisms involved in tumor development in the gastrointestinal tract.
IPS Cell Modeling of Hepatic Differentiation
Several projects in the lab utilizes the liver differentiated progeny of iPSC to study genetic and infectious diseases affecting the liver. We have recently started a collaboration with the BU NEIDL to study Ebola virus entry and replication utilizing human iPSC-derived hepatocytes. By accessing primary human target cells we hope to establish a new paradigm in the use of iPSC-derived cells for infectious diseases.
IPS Cell Modeling of Immune/Inflammatory Cell Differentiation
Our long standing interest in the immune system now focuses in the utilization of iPSC-derived immune cells, including myeloid dendritic cells (mDCs), T cells and others as the basis to study several inflammatory diseases such as lupus erythematosus and the signaling cascade responsible for immune dysregulation. Dendritic cells play a central role in the modulation of the immune response and several inflammatory diseases have been shown to have altered mDC function. We aim at generating iPSC containing mutations in IRF5, Myd88, UNC93, TLRs and other relevant genes to test their role in mDC function compared to normal cells. Another project focuses on the generation of T regs and their potential use as modulators of immune tolerance in organ transplantation. We are combining novel engineered stromal cells with gene editing to explore a way to direct the differentiation of iPSC into hematopoietic progenitors and into a T cell/T reg lineage.
Hematopoietic Stem Cells
Hematopoietic Stem Cells (HSCs) are the most thoroughly characterized stem cell population in the body and their study has resulted in well established methods for their isolation, purification and reliable assays of HSC function. During the last few years we have substantially improved our ability to genetically manipulate HSCs using viral vectors for gene transfer. Despite these efforts, few genes are known to play a role in the processes of stem cell self-renewal and differentiation. Understanding the molecular mechanisms that govern those unique functions are crucial for developing the promise that stem cells hold for developmental biology and regenerative medicine.
3D Bioprinting
A novel exciting area of research in the lab is the use of human iPSC for the 3D reconstruction of liver and gut organs. For this purpose we have recently acquired a new 4th generation Bioplotter to test new biomaterials for the establishment of functional miniorgans combining differentiated cells, such as hepatocytes, intestinal and endothelial cells.