Wetzler Laboratory
Lee M. Wetzler, MD
Professor
Education:
MD – SUNY/Upstate Medical Center
General field of research:
Immunology, Vaccine Development, Bacterial Pathogenesis and Innate Immunity
Affiliations other than medicine:
Evans Center for Interdisciplinary Biomedical Research
Dept. of Microbiology
Immunology Training Program
Contact information:
Office
EBRC 637
Phone: 617-414-4394
Lab
Fax: 617-414-5280
lwetzler@bu.edu
Research Group information:
Michael Reiser PhD, Postdoctoral Fellow, mr311@bu.edu
Ian Francis, Graduate Student, ianpfran@bu.edu
Christina Lisk, Graduate Student, clisk@bu.edu
Rachel Yuen, Graduate Student, rryuen@bu.edu
Xiuping Liu, Technician, Xiuping.Liu@bmc.org
Keywords:
Vaccines, Adjuvants, Pathogenesis, Innate Immunity, Neisseria
Summary of Research Interest:
Our laboratory investigates innate and adaptive immunity and microbial pathogenesis, especially in regards to vaccine development. One major aspect of this work centers on the pathogenic Neisseria, Neisseria gonorrhoeae and Neisseria meningitidis. We have found that the major outer membrane protein of these organisms, the Neisserial porin PorB, can work as an immune adjuvant due to it recognition by the pattern recognition receptor TOLL-like receptor (TLR) 2. We have found that antigen presenting cells, including B cells, dendritic cells and macrophages, are activated by PorB in a TLR2, TLR1 and MyD88 dependent manner, inducting upregulation of class II MHC, costimulatory molecule CD86 and other markers of activation. Moreover, MAPK signaling events are required for the upregulation of the expression of these markers, as well as production of pro-inflammatory cytokines.
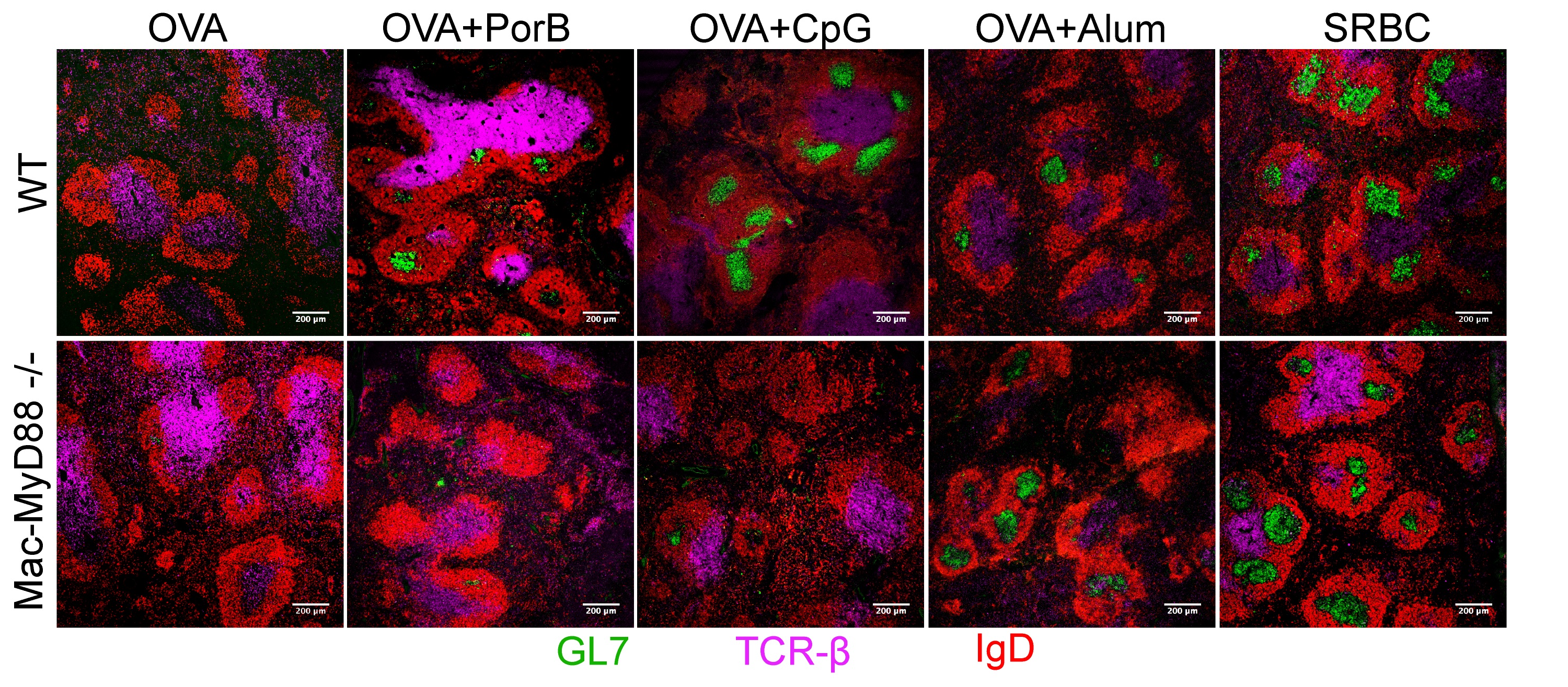
We are investigating the use of this TLR2 ligand, PorB, as a vaccine adjuvant using classic antigens i.e. OVA, and more relevant antigens, i.e. bacterial capsular polysaccharide. We are also comparing the PorB adjuvant activity to other classical and investigative adjuvants including Alum, MF59, CpG DNA and MPL. We have shown that PorB adjuvant activity is absolutely MyD88 dependent and also largely dependent on the presence of TLR2 (even though there is still some adjuvant activity in TLR2 KO mice). Utilizing conditional KO mice (using the Cre-Lox system) we have discerned that MyD88 signaling in B cells, Dendritic Cells (DC) and Macrophages are essential for the PorB adjuvant activity. Surprisingly, the presence of MyD88 in macrophages seems to be the most essential component needed for this adjuvant activity. Investigations are ongoing in this area. Moreover, we have shown that PorB can enhance both antigen uptake and antigen presenting cell trafficking (especially DCs and macrophages) and these effects are mainly TLR2 dependent. We have recently begun investigating the effect of PorB on germinal center formation and have found that this molecule is extremely potent in inducting germinal centers and proliferating B cells. Interestingly, if MyD88 is conditionally KO’d in macrophages, these germinal center findings are abrogated. Using these same mice we have found that PorB induces both Th1 and Th2 type responses as determined by cytokine profiles and IgG isotypes. PorB induced a greater breadth of response in this regard as compared to Alum, MF59 and MPL. We are currently investigating the mechanisms behind these findings.
We have also begun a systems immunologic analysis of the adjuvant activity of PorB. We have found that PorB induces a genetic program consisting of genes and gene sets needed for immunoglobulin synthesis and cellular proliferation after only one or two immunizations as opposed to a greater number of immunizations of antigen alone to induce a similar response. We are still investigating the genes induced and shall compare these results to results obtained with other adjuvants (both TLR dependent and independent) to determine if these results can be used to correlate with different responses induced by various adjuvants (i.e. Th types, IgG isotypes etc., as mentioned above). This also revealed that PorB can induce certain profiles of micro RNAs that may also be associated with its immune stimulating properties and we are in the midst of studies to further address these interesting findings.
A significant new set of studies currently being performed win collaboration with Dr. Scott Gray-Owen at the University of Toronto is the examination of a new murine model for gonococcal infections and the use of this model to examine potential anti-gonococcal vaccine candidates. Dr. Gray-Owen has developed transgenic mice that express human gonococcal adhesins and other molecules that enhance gonococcal pathogenesis, that are normally lacking in mice. These include CEACAMs, complement binding proteins and iron binding proteins. These mice mimic human infection to a much greater extent as compared to wild type mice, leading to a superior model to both study gonococcal pathogenesis and immunity. We have been able to demonstrate that at early time points more inflammation and cytokine induction occurs in these transgenic mice as opposed to other commonly used models, demonstrating that these previously used models my not be the best approach to study gonococcal infections. These studies are currently on going.
Representative Publications:
- Platt, A, Wetzler L. M. 2013 Innate immunity and vaccines. Curr Top Med Chem. 2013;13(20):2597-608. PubMed PMID: 24066890.
- Platt, A., Macleod, H., Massari, P., Liu, X., Wetzler, L.M., 2013 In Vivo and In Vitro Characterization of the Immune Stimulating Activity of the Neisserial Porin PorB. PLoS One. 2013 Dec 11;8(12):e82171. doi: 10.1371/journal.pone.0082171. PubMed PMID: 24349212.
- Wetzler LM, Feavers IM, Gray-Owen SD, Jerse AE, Rice PA, Deal CD. 2016 Summary and recommendations from a National Institute of Allergy and Infectious Diseases (NIAID) workshop on “Gonorrhea Vaccines: the Way Forward”. Clin Vaccine Immunol. Jun 22. pii: CVI.00230-16. [Epub ahead of print] PubMed PMID: 27335384.
- Xu F, Reiser M, Yu X, Gummuluru S, Wetzler L, Reinhard BM. 2016 Lipid-Mediated Targeting with Membrane-Wrapped Nanoparticles in the Presence of Corona Formation. ACS Nano. Jan 26;10(1):1189-200. doi: 10.1021/acsnano.5b06501. Epub 2016 Jan 6. PubMed PMID: 26720275; PubMed Central PMCID: PMC4842014.
- Islam EA, Shaik-Dasthagirisaheb Y, Kaushic C, Wetzler LM, Gray-Owen SD. 2016 The reproductive cycle is a pathogenic determinant during gonococcal pelvic inflammatory disease in mice. Mucosal Immunol. Jul;9(4):1051-64. doi: 10.1038/mi.2015.122. Epub 2015 Dec 23. PubMed PMID: 26693700; PubMed Central PMCID: PMC4915993.
- Reiser ML, Mosaheb MM, Lisk C, Platt A, Wetzler LM. 2017 The TLR2 Binding Neisserial Porin PorB Enhances Antigen Presenting Cell Trafficking and Cross-presentation. Sci Rep. 2017 Apr 07; 7(1):736. PMID: 28389664.
- Mosaheb MM, Reiser ML, Wetzler LM. 2017 Toll-Like Receptor Ligand-Based Vaccine Adjuvants Require Intact MyD88 Signaling in Antigen-Presenting Cells for Germinal Center Formation and Antibody Production. Front Immunol. 2017; 8:225. PMID: 28316602