FAQs – Quality Assurance Reviews
One of the primary activities of the Research Quality Program (RQP) is to conduct Quality Assurance (QA) reviews of selected IRB approved research studies. If you have received a notice informing you that your study has been chosen to undergo a QA review, the following FAQs will provide you with additional information about the process. Review section 11.3 of the BMC/BUMC HRPP policies and procedures for further details.
- Is this a for-cause/targeted audit?
No, a QA review is not a for-cause/targeted audit and is not performed in response to a concern or complaint. Instead, QA reviews are routinely performed for any study that has been recently IRB-approved and meets the criteria for conducting a QA review. QA reviews are intended to provide a quick “snapshot” overview of the study’s compliance with regulations, policies, and best practice guidance.
- Who will conduct the QA review?
QA reviews are conducted by Human Research Quality Managers from the Research Quality Program (RQP), under the BMC/BU Medical Campus Office of Human Research Affairs (OHRA). While we are a part of BMC/BUMC’s research compliance, we are separate from the IRB.
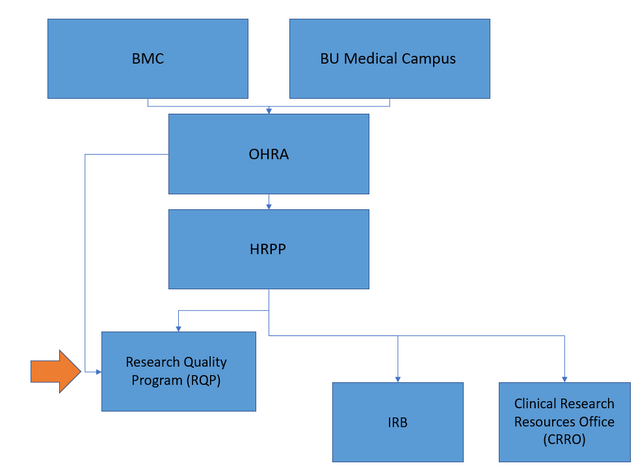
- Why was my study selected for a QA review?
Your study was selected because it was recently approved by the BMC/BUMC IRB. We try to identify studies shortly after IRB-approval once at least a few subjects have been enrolled so that any issues that need to be corrected are identified early in the conduct of the study. However, a study may undergo a QA review at any time during the study lifecycle. Any study can be chosen, but the emphasis is on those studies that meet one or more of the following characteristics:
-
- Greater than minimal risk; or
- Investigator-initiated; or
- Interventional clinical trials; or
- First time Principal Investigators; or
- Studies where the Principal Investigator holds the IND or IDE; or
- Studies with a conflict of interest management plan; or
- Multi-site studies where the Principal Investigator has the responsibility for overseeing all sites; or
- Other characteristic indicating that a QA Review would be appropriate
- Do I still need a QA review if the study sponsor sends a monitor to do this kind of activity?
The sponsor has a regulatory obligation to ensure proper monitoring of the study. Similarly, the BMC/BUMC HRPP is responsible for evaluating and improving the conduct of research it oversees. Consistent with this responsibility, we may perform a QA review for the research activities of any investigator at BMC/BUMC, regardless of monitoring activities performed by the sponsor.
- What is the purpose of this review?
QA reviews are performed to ensure that the rights and welfare of study participants are protected. QA reviews of new and ongoing research studies ensure that IRB-approved research is conducted in compliance with applicable federal and state regulations, BMC/BUMC HRPP institutional policies, policies of the IRB of record (if ceded), and other guidance. QA reviews are intended to be educational and consultative in nature. The goal is to identify and help correct potential problems in study conduct, documentation, or process before they affect safety of participants and/or validity/integrity of study data.
- What does the QA review entail?
The QA review will take place either remotely or require an on-site visit based on the format of source documents (paper, electronic or both). The QA review typically focuses on study documents such as: the original signed informed consent forms; subject eligibility assessments; study visit activities; compliance with the IRB approved confidentiality plan and the data and safety monitoring plan; and staff qualifications, training, and delegation of responsibilities. Other aspects of the study may also be reviewed as needed.
- How much time does a QA review take?
Whether remote or on-site, we will meet with you for 20-30 minutes on the day of the review to provide an overview of the QA review process. The Principal Investigator is not required to take part in the initial QA meeting as long as a knowledgeable member of the study team attends. Most on-site visits, if required, are completed in less than four hours. After the initial QA meeting and providing us with study documents, the study team does not have to be present for the remainder of the on-site visit.
- How do I prepare for a QA review?
Whether your study is undergoing an on-site or remote review, you should prepare by reserving 20-30 minutes of your time for the initial meeting at the time of the on-site visit or scheduled video call. Additionally, please complete any requests we make for electronic study document sharing ahead of the meeting. If your study is undergoing an on-site review, please reserve a private space for us to review your documents and ensure that documents are readily available at the start of the QA review visit. Beyond this, you do not need to do anything to prepare for the QA review. The best approach to the review is to present your study documents “as-is” so that we can identify any existing issues/areas for improvement and help you to address them.
- How will I find out the results of the QA review?
Once the review is complete, a QA review report will be emailed to the Principal Investigator and study staff involved in the QA review. The report will detail findings which are categorized as Actionable Findings (major deviations, minor deviations, and Important Findings) or Best Practice Recommendations. Actionable Findings will outline the steps you should take to address them, including any applicable reporting requirements (e.g., reporting to the IRB of record, sponsor, or other institutional requirements). Best practice recommendations are suggestions for the study team to consider integrating into their processes. They are not requirements.
- Are the review findings shared with any other office/department?
Per BMC/BUMC HRPP policy, the QA review report is shared with the OHRA Director and the BMC Research Compliance Officer. If the study is ceded, the OHRA Director will summarize the findings and provide that information to the point of contact at the IRB of record. Additionally, the Principal Investigator is responsible for sharing any reportable findings with the appropriate personnel as indicated in the report (e.g., the sponsor). The Principal Investigator may also share the QA report with anyone they would like to.
If you have any additional questions regarding the OHRA Quality Assurance Review process, please feel free to contact the OHRA Human Research Quality Manager(s):
Alyssa Pingitore: aping@bu.edu or Marena Neggers: mneggers@bu.edu