Monitoring and Reporting
Data Safety and Monitoring Plan
- No longer a separate template; instead, use the Protocol Template
Corrective and Preventative Action (CAPA) Plan
- Click here for a CAPA plan template
Reporting table for Unanticipated Problems, Adverse Events, Serious Adverse Events, and Deviations
- Click here for a table showing required reporting to the IRB after initial approval.
- Note that if a different IRB is the IRB of record:
- The reporting requirements of the IRB of record must be followed; AND
- Internal Study Personnel Changes and local Unanticipated Problems must also be reported to the BMC-BU Medical Campus IRB
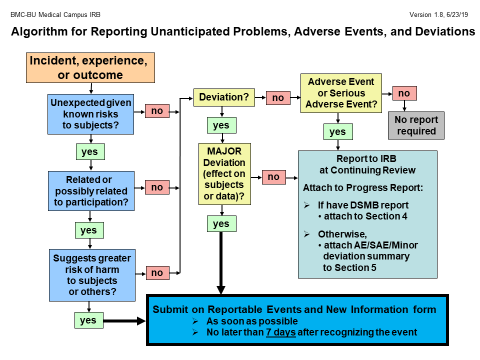
Flow Chart to determine Unanticipated Problems and Adverse Events
- Click here for a copy of the reporting Algorithm or see below: