Induced Pluripotent Stem (iPS) Cell Bank
Induced Pluripotent Stem (iPS) Cell Bank: An International Resource for Investigators studying Diseases Affecting the Boston Medical Center Patient Population
INITIATION DATE:
12.01.09
ARC DIRECTORS AND CO-DIRECTORS:
Darrell Kotton, PhD; Co-Director; Associate Professor;
Medicine/Pathology
Gustavo Mostoslavsky, PhD; Co-Director; Assistant Professor;
Medicine/Microbiology
Discovery highlight, March 2017:
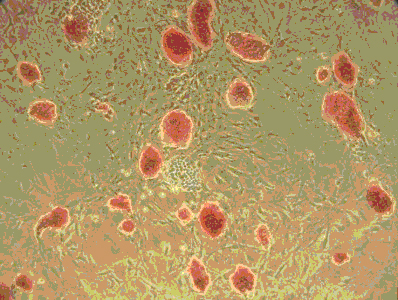
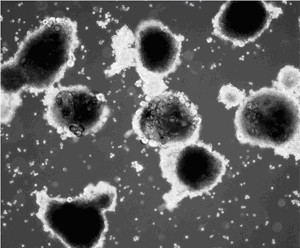
Our ARC initiative, the first one to be awarded at Boston University, facilitated the launch of an Open-Source stem cell bank that has grown over the years into one of the most widely shared NIH-sponsored stem cell repositories in the US. Today this repository continues to serve as the heart of our Center for Regenerative Medicine and has become the largest bank of disease-specific induced pluripotent stem (iPS) cells for studying sickle cell disease, amyloidosis, and genetic lung diseases including alpha-1 antitrypsin deficiency, cystic fibrosis, and a wide variety of genetic neonatal lung disorders. A commitment to international, unrestricted open sharing of cell vials from this repository along with IRB-approved informed consent documentation and associated clinical datasets continues to make this repository, launched with ARC support, a valuable national treasure for stem cell and regenerative medicine research and personalized drug development.
OVERVIEW OF GOALS AND MISSION, 2010:
Abstract
The recent discovery that mature somatic cells can be reprogrammed into induced pluripotent stem (iPS) cells provides unprecedented opportunities to better understand and treat a variety of human diseases. We have recently developed a novel reagent, the lentiviral ‘stem cell cassette’ vector (STEMCCA) that allows the most efficient generation of iPS cells. This ARC project utilizes this vector to develop an extensive bank of human iPS cells from individuals with diseases commonly found in the Boston Medical Center patient population: Sickle Cell Anemia, Amyloidosis, Emphysema, Inflammatory Bowel Disease, Scleroderma, and Diabetes. Cell lineages relevant to each disease phenotype will be generated from iPS cells in culture using methods we have developed that essentially mimick known developmental milestones in vitro in a process termed ‘directed differentiation’. The epithelial, endothelial, and mesodermal cell types generated are expected to recapitulate disease phenotypes in vitro allowing a powerful and limitless platform upon which to model disease pathogenesis and develop novel cell-based therapeutics. The cell bank and methodologies supported by this ARC are thus expected to directly result in programmatic grant proposals from Boston University and to serve as an international resource for investigators interested in modeling the earliest stages of human development.
ARC Members:
| Name/Title | Dept/School | Role in ARC | Web Link | |
| Darrell Kotton, MD; Professor | Medicine/Pulomnary School of MedicineDirector, CReM |
Co-Director | dkotton@bu.edu | http://www.kottonlab.com/ |
| Gustavo Mostoslavsky, MD, PhD; Associate Professor | Medicine/Microbiology School of Medicine |
Co-Director | gmostosl@bu.edu | http://www.mostoslavskylab.com/ |
| George J. Murphy, PhD; Assistant Professor | Hematology Oncology School of Medicine |
API | gjmurphy@bu.edu | http://www.bumc.bu.edu/hematology/ research-activities/george-murphy-phd/ |
| Wellington V. Cardoso, MD, PhD; Adjunct Professor | Medicine/Pathology School of Medicine |
API | wcardoso@bu.edu | http://www.bumc.bu.edu/pulmonary/cardosolab/ |
| Joyce Y. Wong, PhD; Professor | Biomedical Engineering College of Engineering |
API | jywong@bu.edu | http://people.bu.edu/wonglab/ |
MAIN ARC PROJECT(S) FOR 2009-2010:
iPS cells are theoretically easily obtained from virtually any human being. Only recently, however, have methods become available to generate ‘clinical grade’ iPS cells that are free of any residual reprogramming transgenes. The generation of transgene-free iPS cells is particularly important in order to minimize the oncogenic potential of iPS cells, and the residual presence of reprogramming transgenes in iPS cells can adversely affect the capacity of iPS cells to undergo directed differentiation in vitro. Thus, after the removal of any residual reprogramming transgenes, the opportunity now exists to direct the differentiation of human iPS cells in culture into mesendodermal lineages that are putatively identical to those derived from the current gold-standard of pluripotent stem cells, ES cells.
Several fundamental questions now must be addressed in order to advance the field: 1) How closely do the differentiated cell types derived from ‘clinical grade’ iPS cells in vitro resemble those cells generated from either ES cells or from primary tissues? 2) How much variability exists in the differentiation capacity of different iPS cell clones derived from the same individual, and, if significant variability exists, can screening methods in vitro rapidly identify the ‘best’ clone able to form a specified lineage? 3) What effects will inherited disease-causing mutations have on the phenotype of iPS cells, either in the undifferentiated state or after differentiation into the relevant lineages required to treat/model diseases?
ARC AS A RESOURCE:
For more information about resources provided to the BUMC campus by this ARC, please click on the following link which details core facilities and protocols developed by these ARC investigators: www.bumc.bu.edu/stemcells