Cardiovascular Consequences of Metabolic Disease
INITIATION DATE
12.01.09
ARC DIRECTORS AND CO-DIRECTORS:
Kenneth Walsh, PhD; Professor; Medicine
Richard Cohen, MD, Professor, Medicine
OVERVIEW OF GOALS AND MISSION:
Abstract
Obesity promotes a chronic inflammatory state, which contributes to the development of insulin resistance and cardiovascular disease. This ARC is intended to promote translational research by bringing basic and clinical research laboratories together to study the cardiovascular consequences of obesity and diabetes. This ARC will coordinate activities of basic science labs and clinical research labs to assess the interrelationship between metabolic dysfunction and vascular disease.
ARC Members:
| Name/Title | Dept/School | Role in ARC | Web Link | |
| Kenneth Walsh, PhD; Prof | Medicine/ Whitaker Cardiovascular Institute | Director | kxwalsh@bu.edu | http://www.bumc.bu.edu /medicine/walsh/ |
| Noriyuki Ouchi, MD, PhD; Assist Prof | Medicine/ BUSM | ARC member | nouchi@bu.edu | |
| Noyan Gokce, MD; Prof | Medicine/ BUSM |
ARC member |
Noyan.Gokce@bmc.org | http://profiles.bu.edu/Noyan.Gokce |
| Joseph Vita, MD; Prof | Medicine/ BUSM | ARC Project Investigator | ||
| Paul Pilch, PhD; Prof | Biochemistry/ BUSM | ARC member | ppilch@bu.edu | http://profiles.bu.edu/Paul.Pilch |
| Richard A. Cohen, MD; Prof | Medicine/Vascular Biology |
ARC Co-Director |
||
| Jane Freedman, MD; Prof | Medicine/ Cardiology | ARC member | ||
| Kahraman Tanriverdi, PhD; Res Assist Prof | Medicine/ BUSM | ARC member | ||
| Stephen Farmer, PhD; Prof | Biochemistry/ BUSM | ARC member | sfarmer@bu.edu | http://profiles.bu.edu/Stephen.Farmer |
| Mengwei Zang, MD, PhD; Asst Prof | Medicine/Vascular Biology | ARC member | mwzang1@bu.edu | http://www.bumc.bu.edu /medicine/faculty/zang/ |
| Konstantin Kandror; Prof | Medicine/Biochemistry | ARC member | kkandror@bu.edu | http://profiles.bu.edu/Konstantin.Kandror |
| Tamar Aprahamian; Asst Prof Med | Medicine/Renal Section | ARC member | aprahami@bu.edu | http://www.bumc.bu.edu/medicine/faculty/aprahamian/ |
| Neil Ruderman MD; Prof | Medicine/ Diabetes and Metabolism | ARC member | nrude@bu.edu | http://profiles.bu.edu/Neil.Ruderman |
| Wilson Colucci MD; Prof | Medicine/Cardiovascular Medicine | ARC member | wcolucci@bu.edu | http://profiles.bu.edu/Wilson.Colucci |
MAIN ARC PROJECT(S) FOR 2009-2010:
We propose projects to define the molecular mechanisms by which metabolic disease impacts vascular function and vice versa. The projects involve genetic, physiological and clinical studies that focus on the central theme of defining how obesity and insulin resistance lead to biochemical changes in endothelial cell that impair function and promote inflammation. The ARC takes a well integrated approach to understand the functional interrelationships between metabolic disease and vascular dysfunction. Importantly, the ARC is translational, and it strives to meaningfully involve mechanistic studies in experimental systems with measures of vascular cell function in patients.
A number of novel hypotheses will be evaluated by the ARC: 1) The metabolic-regulatory and vascular-protective actions of polyphenols (i.e. resveratrol) are mediated, in part, by their ability to upregulate adiponectin expression in a SIRT1-dependent manner. 2) Polyphenols improve vascular function in patients with T2DM. 3) Vascular dysfunction within adipose tissue leads to inflammation and metabolic dysfunction.
ARC AS A RESOURCE:
The funds from this ARC will be used to 1) initiate collaborative studies in mouse models on mechanisms of obesity-linked cardiovascular diseases and 2) support research where basic science and clinical research labs perform molecular analyses on tissues and cell samples isolated from patient populations. A goal is to foster cross-talk between basic and clinical labs by establishing an environment where techniques employed in mouse studies are used to characterize clinical specimens at a deeper mechanistic level.
Pictures, Images, Figures:
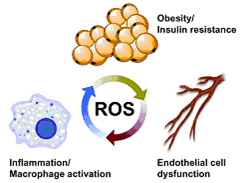
Obesity and associated metabolic abnormalities are becoming major health care concerns around the world. Currently it is estimated that more than 60% of adults and 30% of children are overweight in the United States, and if trends continue it is estimated that more than 50% of the world’s adults will be overweight or obese by 2030. Obesity and its comorbidities, including hypertension, hyperlipidemia and type 2 diabetes mellitus, have a devastating impact on vascular function leading to conditions that promote cardiovascular disease. Due in large part to the increased prevalence of cardiovascular disease, it is estimated that life expectancy declines by 3 years for those with a Body Mass Index (BMI) over 30 and 10 years for those with a BMI over 40.
Endothelial cell function has long been viewed as a barometer of overall cardiovascular health. Endothelial dysfunction refers to a state where endothelial cells lose the ability to produce bioactive nitric oxide and increase their expression of vasoconstrictor, pro-inflammatory and pro-thrombotic factors. In human subjects, studies have shown that endothelial dysfunction, recognized as impairments in flow-mediated dilation or endothelial-dependent vasodilation in response to acetylcholine, is predictive of cardiovascular events in patients with hypertension, coronary artery disease, peripheral artery disease, etc. Of relevance to the theme of the ARC, Vita and Gokce laboratories have shown that obesity is associated with impaired endothelial function and that elevated BMI correlates with blunted flow-mediated dilation of the brachial artery. Conversely, weight loss is associated with improvements in this parameter. Experimental studies have documented that “oxidant stress” is central to the development of endothelial dysfunction. Oxidant stress refers to a condition where reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell. This pathological state contrasts with normal endothelial cell homeostasis where low levels of ROS, particularly nitric oxide and hydrogen peroxide, act as mediators of cell signaling and are required for normal endothelial cell function.
While endothelial cell dysfunction is a major contributor to the development of diverse cardiovascular diseases, recent findings suggest that it may also contribute to the inflammatory processes within adipose tissue that promote metabolic dysfunction. It is widely recognized that the vascular endothelium is both affected by and contributes to inflammatory processes. Obesity leads to the downregulation of anti-inflammatory factors, such as adiponectin, that pacify the vascular endothelium, and the upregulation of pro-inflammatory factors that activate endothelial cells and promote a dysfunctional phenotype. In turn, the activated endothelium expresses adhesion molecules and chemotactic factors that accelerate and localize inflammatory processes. Although poorly understood at this time, this yin-yang relationship between the vascular endothelium and inflammation is likely to have a considerable impact on the metabolic properties of adipose tissue. Recently, a number of studies have pointed to the importance of the microvasculature in controlling inflammation in the fat pad and overall metabolic function. Studies with obese mice have documented that the fat pads of these animals display capillary rarefaction and evidence of hypoxia. Consistent with these findings, recent work from Gokce and colleagues has shown that macrophage filtration and the presence of crown-like structures of macrophages around necrotic adipocytes is associated with metabolic dysfunction and impaired endothelium-dependent flow mediated vasodilation in obese subjects. Collectively, these findings have led to the hypothesis that ischemic stress in the fat pad initiates the death of adipocytes which, in turn, leads to the recruitment and activation of macrophages that are the hallmark of “inflamed” fat. Therefore, the status of endothelial cell function may have an integral role both in mediating the effects of metabolic disease on the cardiovascular system and in controlling the metabolic state of the organism by influencing, either positively or negatively, the While endothelial cell dysfunction is a major contributor to the development of diverse cardiovascular diseases, recent findings suggest that it may also contribute to the inflammatory processes within adipose tissue that promote metabolic dysfunction. It is widely recognized that the vascular endothelium is both affected by and contributes to inflammatory processes. Obesity leads to the downregulation of anti-inflammatory factors, such as adiponectin, that pacify the vascular endothelium, and the upregulation of pro-inflammatory factors that activate endothelial cells and promote a dysfunctional phenotype. In turn, the activated endothelium expresses adhesion molecules and chemotactic factors that accelerate and localize inflammatory processes. Although poorly understood at this time, this yin-yang relationship between the vascular endothelium and inflammation is likely to have a considerable impact on the metabolic properties of adipose tissue. Recently, a number of studies have pointed to the importance of the microvasculature in controlling inflammation in the fat pad and overall metabolic function. Studies with obese mice have documented that the fat pads of these animals display capillary rarefaction and evidence of hypoxia. Consistent with these findings, recent work from Gokce and colleagues has shown that macrophage filtration and the presence of crown-like structures of macrophages around necrotic adipocytes is associated with metabolic dysfunction and impaired endothelium-dependent flow mediated vasodilation in obese subjects. Collectively, these findings have led to the hypothesis that ischemic stress in the fat pad initiates the death of adipocytes which, in turn, leads to the recruitment and activation of macrophages that are the hallmark of “inflamed” fat. Therefore, the status of endothelial cell function may have an integral role both in mediating the effects of metabolic disease on the cardiovascular system and in controlling the metabolic state of the organism by influencing, either positively or negatively, the microenvironment within the fat pad. Therefore, a premise of this proposal is that obesity favors a vicious cycle whereby endothelial cell dysfunction in the fat pad leads to metabolic dysfunction, reflected by adipocytokine dysregulation, adipocyte necrosis and inflammation. In turn, metabolic dysfunction promotes endothelial cell dysfunction, both in the fat pad and the systemic circulation, putting further stress on the adipocyte.