Obesity, Cancer and Inflammation
INITIATION DATE
12.01.10
ARC DIRECTORS AND CO-DIRECTORS:
Dir. Gerald V Denis, PhD
Dept Medicine
Associate Professor, Cancer Center
gdenis@bu.edu
617-414-1371
Co-Dir. Barbara Nikolajczyk, PhD
Dept Microbiology
Associate Professor
bnikol@bu.edu
617-638-7019
Discovery highlight, March 2017:
Why is obesity only weakly associated with certain ‘obesity-driven’ cancers?
Recent population studies identify cohorts of high BMI subjects with unexpectedly reduced risk for breast and colon cancer, and normal BMI subjects with unexpectedly elevated risk for breast cancer, provoking hard thinking about cellular and molecular mechanisms that most strongly couple obesity to cancer occurrence or progression. Emerging work suggests that abnormal metabolism and its associated chronic inflammation make the difference. Type 2 diabetes, for example, is a chronic inflammatory disease with specific imbalances in T cell and myeloid-origin cytokines. Inflammation is elevated systemically, measured through blood biomarkers, and locally in adipose tissue. Cytokines and chemokines likely modify tumor microenvironments in dangerous ways. High BMI subjects with low inflammation and less disturbed metabolism appear to have reduced risk for certain obesity-associated cancers, whereas lean or slightly overweight subjects with high inflammation and metabolic abnormalities have elevated risk. This latter phenotype is prevalent among South Asian adults, and suggests we are not monitoring certain normal weight adults sufficiently for risks of ‘obesity-associated’ cancers. Profiling of patient metabolism and inflammation should accompany measures of body composition when considering cancer risk; the evidence base for these refinements must be extended through new, prospective observational studies.
Our discoveries now support a new hypothesis: metabolic disease in obesity promotes breast cancer incidence and metastasis. Interestingly, metabolic complications and inflammation arise in South Asian adults at much lower BMI values than in U.S. adults. An innovative problem thereby emerges: it is unknown whether Type 2 diabetes in obese patients, a common phenotype in the U.S., is the same disease with respect to breast cancer progression as Type 2 diabetes in lean patients, who are common in India. Available cohorts of lean or obese patients with or without diabetes and with or without cancer suggest that analysis of pooled data from U.S. and Indian studies that might reveal new mechanism and associations.
At present, the infrastructure for data sharing of clinical, molecular and cellular datasets between Indian and U.S. institutions is underdeveloped. Our team is currently engaged in the development of novel software to permit scientific research, while sensitive data do not leave each host institution’s protected environments, minimizing the risk for unwarranted exposure of private data. Our immediate goal is to construct a prototype software infrastructure that (1) enables collaboration between researchers across institutions in India and the U.S., (2) allows investigators to establish common representation formats, (3) supports securely performing analyses over joint data sets using secure Multi-Party Computation (MPC) techniques, and (4) enables sharing and dissemination of research results. MPC is a cryptographic technique that makes it possible to perform statistical analyses on a collection of sensitive medical and health data sets that are owned and hosted by distinct data contributors, without requiring that data be shared in the clear or revealing anything except the result of the analysis. Our long term goal is to promote data sharing among Indian institutions, and between U.S. and Indian investigators.
The public health need for this research is high, given the large numbers of adults in Asia who will be affected, as well as Americans of Asian origin. Our global health partners at Boston University have been enthusiastic supporters of this team science initiative and the impact of our results is growing rapidly. We continue to seek substantial Federal or foundation funding to develop this research.
OVERVIEW OF GOALS AND MISSION, 2010-2012:
Abstract
In diet-induced obesity, human adipose tissue is in a chronic state of inflammation, with systemic consequences. This state is implicated in the emergence of metabolic syndrome, insulin resistance, dyslipidemia and Type 2 diabetes, as well as serious co-morbidities, including cardiovascular disease and cancer. However, roughly 25% of the adult obese population are ‘metabolically healthy’ and protected from inflammation-driven complications; novel mouse model studies support the view that reduced inflammatory signaling decouples obesity from diabetes and cancer, but this idea has never been tested in humans. The central hypothesis of this ARC is that ‘metabolically healthy obese’ individuals are also protected from obesity-associated cancers. The rationale for the proposed research is that new therapeutic interventions for cancer and T2D will be developed, that already-approved anti-obesity drugs will be shown to have anti-cancer value, and better biomarkers will be developed to identify obese patients at elevated risk for cancer. We will develop two Specific Aims: 1. Use the Framingham dataset to evaluate retrospectively cancer incidence as a function of metabolism and inflammatory markers. 2. Determine inflammatory profiles of obese patients who are metabolically healthy, well-matched to unhealthy obese controls; to develop obesity-associated cancer biomarkers and ‘druggable’ targets.
ARC Members:
| Name/Title | Dept/School | Role in ARC | Web Link | |
| Gerald V. Denis PhD ; Associate Professor | Cancer Center/Dept. Medicine, BUSM ;Dept. Pharmacology, BUSM ; Immunology Training Program, BUSM; Program in Molecular Medicine, BUSM; Member, Boston Nutrition Obesity Research Center (BNORC) | Director | gdenis@bu.edu | http://profiles.bu.edu/Gerald.Denis |
| Caroline M. Apovian, MD, FACP, FACN; Professor | Director, Nutrition and Weight Management Center, BMC; Co-Director, Human Metabolic and Genetic CORE, BNORC; Associate Professor of Pediatrics, BUSM; Associate Director, Graduate Program in Medical Nutrition Sciences, BUSM | API | Caroline.Apovian@bmc.org | http://www.bumc.bu.edu/medicine/faculty/nutritionresearch/ |
| Deborah J. Bowen, PhD; Adjunct Professor | Community Health Sciences, BU SPH | API | dbowen@bu.edu | http://profiles.bu.edu/Deborah.Bowen |
| Susan K. Fried, PhD; Adjunct Professor | Endocrinology, Diabetes & Nutrition, Dept. Medicine, BUSM | Member | skfried@bu.edu | http://profiles.bu.edu/Susan.Fried |
| Konstantin Kandror, PhD; Professor | Dept. Biochemistry, BUSM; Member, BNORC | Member | kkandror@bu.edu | http://profiles.bu.edu/Konstantin.Kandror |
| Lynn L. Moore, Dsc; Associate Professor | Dept. Preventive Medicine and Epidemiology, BUSM | API | llmoore@bu.edu | http://profiles.bu.edu/Lynn.Moore |
| Barbara S. Nikolajczyk, PhD ; Associate Professor | Dept. Microbiology, BUSM; Immunology Training Program, BUSM; Member, BNORC | Co-
Director |
bnikol@bu.edu | http://profiles.bu.edu/Barbara.Nikolajczyk |
| Paola Sebastiani, PhD; Professor | Dept. Biostatistics and Bioinformatics, BUSPH | API | sebas@bu.edu | http://people.bu.edu/sebas/ |
| Christopher Still, DO; Fellow of the American College of Nutrition; Fellow of the American College of Physicians | Director, Geisinger Obesity Institute, Danville, PA | API | https://webapps.geisinger.org/articles/articles/ChristopherStillDONamedD7457.html |
ARC AS A RESOURCE
Three interest clusters in obesity, cancer and inflammation have been meeting repeatedly over several years.
Obesity: Drs Denis, Apovian, Fried, Kandror & Nikolajczyk meet weekly on Tuesday mornings at 10 AM at BNORC seminars, where they actively discuss obesity, insulin resistance, T2D and inflammation. Apart from Dr Nikolajczyk, they are all also members of Dr Fried’s ARC on sex differences in adipose tissue remodeling. Drs Denis, Apovian, Fried & Still recently attended the Annual Meeting of the Obesity Society in San Diego (10/8/2010-1012/2010), where they met and avidly discussed this and related projects. Dr Apovian brokered the introduction of Dr Still. Dr Fried was the scientific chair of the Meeting. Dr Fried collaborates on Dr Nikolajczyk’s R21 and BADERC grants. Drs Apovian & Nikolajczyk recently co-authored a J. Immunol paper, and Dr Apovian is funded as a collaborator on Dr Nikolajczyk’s recently scored R01 grant. Cancer: Drs Denis & Bowen have known each other since 2005 when they served together as NCI reviewers on the TREC initiative. They have met twice to discuss this project on 7/23/2010, and 9/15/2010 with Dr Fried. Drs Denis & Sebastiani have collaborated since 2006 on bioinformatics analysis of genome-wide transcriptional profiling data in cancer. Inflammation: Drs Denis, Apovian, Fried & Nikolajczyk actively collaborate with other BNORC members to identify and characterize leukocyte infiltrates of human fat obtained from bariatric surgeries. They have jointly generated Preliminary data for recent R01 grant applications. Drs Denis & Apovian jointly wrote an R01 several months ago for which they were co-PIs. Drs Denis & Nikolajczyk are members of the Immunology Training Program and meet weekly on Wednesdays at noon at Program seminars. They have published together (2010) and are actively collaborating on the characterization of pro- and anti-inflammatory lymphoid and macrophage populations in human and mouse adipose tissue in diet induced obesity.
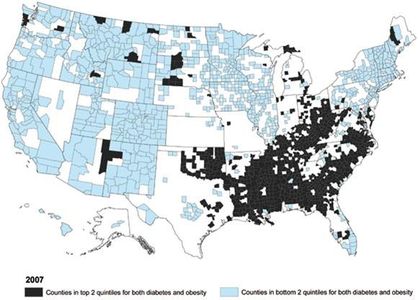
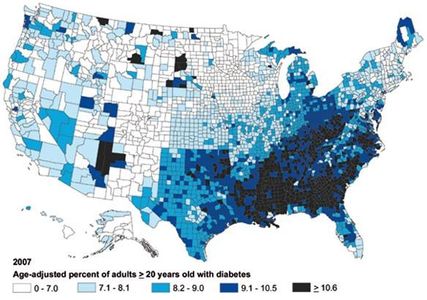
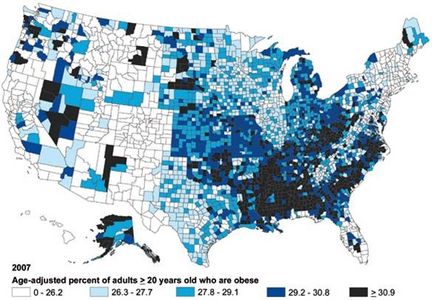
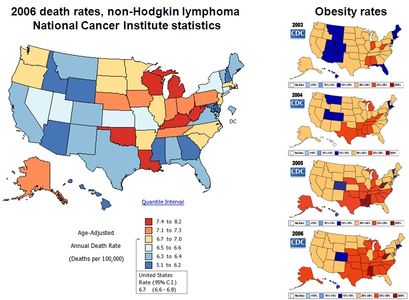
B.1.a. Background. Obesity and its associated co-morbidities are quickly becoming the major health problem of the century [1,2]: the World Health Organization estimates that by 2030, 370 million people worldwide will suffer from obesity-driven Type 2 diabetes (T2D), compared to 177 million in 2000 [3]. Two-thirds of Americans are overweight or obese while about a third of adults (more than 72 million) are obese. Health care costs for obesity-associated T2D, nephrotoxicity, neuropathy, cardiovascular disease (CVD) and hypertension totaled $147 billion in 2008 alone. Furthermore, the American Cancer Society estimated in 2008 that 14-20% of US cancer mortality could be attributed to obesity-associated malignancies, particularly renal, endometrial and colorectal cancers, non-Hodgkin’s lymphoma and post-menopausal breast cancer in women.
Pre-T2D and insulin resistance (IR) in the context of obesity is associated with a chronic state of subclinical inflammation [4], characterized by increased serum concentrations of C-reactive protein [5], interleukin (IL)-6, IL-8, monocyte chemotactic protein (MCP)-1 and tumor necrosis factor (TNF)-α in patients and different animal models of obesity [6-8]. Interestingly, 20–30% of the adult obese population remains relatively ‘metabolically healthy’ despite obesity (MHO) [9]; they display an absence of impaired glucose tolerance, dyslipidemia, hyperuricemia and hypertension [10,11]. Their metabolic and CVD risk profiles are relatively mild [9,12]. The protective mechanism is attributable in part to a reduced inflammatory profile [12], and uncoupling of inflammatory signal transduction from obesity-driven IR [13,14]. In addition, cancer is now considered in part an inflammatory disease. Recent work has identified the mammalian Target of Rapamycin (mTOR) pathway as a potential therapeutic target in cancer treatment. Drugs such as metformin or AICAR improve insulin signaling and mobilize AMP kinase (a cellular energy sensor that negatively regulates mTOR signaling), reducing pro-survival and proliferative signal transduction in renal cancer and chronic myelogenous leukemia [15,16]. Taken together, these data offer strong evidence that molecular pathways link energy metabolism and cancer.
Although the signal transduction events that link inflammation to cancer and IR are intensively studied, the protective phenotype is not well understood. It is not clear if certain alleles in inflammatory loci create a mild hyporesponsiveness in the MHO individual, and thus confer protection. Conversely, certain individuals with a ‘pro-inflammatory phenotype’, who are prone to a cluster of diseases of chronic inflammation (e.g. CVD, Crohn’s disease, rheumatoid arthritis) upon the development of overweight or obesity would be predicted to exhibit higher relative risk for T2D and obesity-associated cancers than the mean risk of the obese population. If the population distribution of inflammatory responses is symmetrical and two-tailed, as many as 18 million Americans could fall in this high-risk cohort, but the relevant biomarkers are unknown. It is crucial therefore to stratify obese populations by inflammatory markers and IR, then compare their cancer incidence.
B.1.b. Rationale. The long term goal of this research is to identify the pathways that regulate cancer risk in obese patients. The short term objectives here are (1) to determine the relationship between inflammation status and cancer incidence in a well-characterized cohort, the Framingham dataset; and (2) to develop flow cytometry-based immunological measures of adipose tissue inflammation in obese patients to establish biomarkers of risk. The central hypothesis is that metabolically healthy obese patients show a reduced incidence of obesity-associated cancers compared to metabolically unhealthy obese patients. This hypothesis is formulated on the basis of (i) Brd2 hypomorph mouse studies, in which decoupled inflammatory signal transduction protects extremely obese animals from IR and T2D as well as from cancer [13], (ii) preliminary data establishing that Brd2 levels in human adipose tissue are inversely correlated with PPARγ levels and thus with inflammation, and (iii) evidence that metabolic/nutrient signaling through mTOR controls cancer progression [15,16]. The rationale for the proposed research is that new therapeutic interventions for both cancer and T2D will be developed, that already-approved anti-obesity drugs will be shown to have anti-cancer value, and better biomarkers will be developed to identify obese patients at elevated risk for cancer.
Aims. The approach will develop two Specific Aims: 1. Use the Framingham dataset to evaluate retrospectively cancer incidence as a function of metabolism and inflammatory markers. Despite intensive study, this cohort has not been evaluated for this correlation. 2. Determine inflammatory profiles of obese patients who are metabolically healthy, matched to unhealthy obese controls. Patients will be well-matched by all available clinical parameters. Brd2 and mTOR signaling will be measured along with leukocyte infiltration of primary adipose tissue in patient biopsies. The expected outcomes of the ARC effort will be to bring together immunologists (Denis, Nikolajczyk), obesity researchers (Apovian, Denis, Fried, Kandror, Nikolajczyk, Still) and cancer investigators (Bowen, Denis, Moore, Sebastiani) to focus on a shared problem of relevance for the BMC clinical population. The group will engage molecular, cellular and bioinformatic hypothesis testing focused on the co-morbidities associated with obesity (T2D, CVD, cancer) that are not captured within traditional disciplinary boundaries. Positive impact is expected from this effort in the form of jointly authored publications, funded grant applications (R21, R01 and U01) and new knowledge that will have enormous health significance, particularly because of the dramatically worsening obesity crisis and expected steep increase in the incidence of obesity-associated cancers.
B.1.c. Plan. This ARC will bring together several investigators from diverse disciplines that share interest in obesity, immunology and cancer, and have highly complementary experience.
Aim 1 takes primarily an epidemiological and bioinformatics approach. The lead investigators will be Dr Deborah Bowen, an expert in energetics and cancer; Dr Lynn Moore, a Framingham investigator and epidemiologist; and Dr Paola Sebastiani, a biostatistics expert; these investigators will consult with Dr Fried, an expert in obesity and sex/depot differences in adiposity. The Framingham dataset will be queried for already-captured measures of inflammation (serum adiponectin/resistin, C reactive protein, IgE, IL-1β, IL-6, TNFR, MCP-1), insulin function (2-hour insulin, HbA1c, diabetic status), obesity (ratio of hip-to-waist circumference, abdominal fat CT, DEXA) and cancer incidence (all cancers, but with specific focus on renal, endometrial and colorectal cancers, non-Hodgkin’s lymphoma and post-menopausal breast cancer). Initially, the investigation will determine if there is sufficient statistical power to identify an MHO cohort and then compare their cancer rates to the non-MHO cohort, to test the hypothesis that MHO cancer rates are lower.
Aim 2 takes primarily a cellular and biochemical approach. The lead investigators will be Dr Apovian, an expert in obesity, Dr Denis, Dr Nikolajczyk and Dr Still, a bariatric surgeon. The supporting investigators will be Dr Fried and Dr Kandror, an expert in mTOR signaling. Human primary adipose tissue will be isolated by Dr Still and shipped to BUSM. Infiltrating leukocytes will be enumerated with multicolor flow analysis on an LSR II cytometer, using CD11b-APC, CD11c-PE and CD68-FITC for macrophages. T cells will be enumerated with CD4-eFluor450 and CD8-PerCP-Cy5.5. Tregs will be enumerated separately with CD4-eFluor450, CD25-PE and Foxp3-APC. Inflammatory and metabolic parameters above will be measured and correlated with mTOR, PPARγ and Brd2 levels and signaling; as well as with family and individual cancer history. Dr Nikolajczyk and Dr Denis are already developing immunophenotyping of adipose tissue stromal vascular fraction cells. This work will develop an analysis of metabolically healthy obese patients well-matched to unhealthy obese to define biomarkers for inflammation-associated T2D and cancer risk; we will be informed by what we learn in Aim 1 analysis of the Framingham dataset.
B.1.d. Novelty, impact and significance. The National Cancer Institute acknowledged the seriousness of the problem of obesity and cancer with the 2005 issuance of a funding initiative called ‘Transdisciplinary Research in Energetics and Cancer’ (TREC). The NCI committed $15M in total costs for the FY2010 reissue of the initiative and $75M in total costs over a 5-year period. Drs Denis and Bowen served as Reviewers on the first peer review panel, and Dr Bowen is recently returned from service on the 2010 reissue panel. The TREC initiative seeks to foster collaboration across multiple disciplines and includes projects that cover the biology, genomics and genetics of energy balance, obesity and cancer risk. Dr Bowen notes that no TREC investigators are currently pursuing the central hypothesis of this application. The publication of the Brd2 hypomorph model [13], which provoked the central hypothesis, is novel; the hypothesis has never been tested in humans. The mechanisms of inflammatory changes in adipose tissue are not understood in the context of cancer, T2D or CVD; yet the public health significance of this problem is undeniable, considering the rising tide of obesity worldwide and these co-morbidities. The TREC Program Announcement notes that considering the difficulty in reversing obesity and that obesity has been related to the development and course of many cancers, a multi-level or systems approach is required. In addition, children may be particularly at risk; the obesity rate among children has steadily risen and evidence suggests that a majority of overweight children will continue to be obese in adulthood. Similarly, weight gain among survivors of many cancers is common and is associated with poor prognosis. Among high-risk populations for obesity (e.g. non-Hispanic blacks), these populations also experience greater morbidity rates from cancers of the breast and other cancers for which obesity is a risk factor.
Specific goals. As discussed above, an interdisciplinary and systems-based approach is necessary to tackle the proposed hypotheses. Investigators in basic science disciplines of signal transduction, immunology, metabolism and transcription must engage a conversation with clinicians who actually identify and treat obese patients, as well as population geneticists and bioinformaticists who have identified the at-risk and protected (MHO) populations. The design and personnel of the proposed ARC address these needs succinctly. Many of these investigators have established collaborations as evidenced by co-authored publications and funded collaborations on other projects. One of the weaknesses of disease-centered research such as cancer or T2D is that investigators must tailor their grant applications to the specific health goals of the individual Federal institutes. Furthermore, immunologists traditionally do not attend the scientific conferences of obesity researchers, and cancer biologists do not attend cardiology meetings. The need for a more integrative approach is obvious, and the choice of the ARC investigators named here addresses this deficiency.
New Technologies. Dr Sebastiani has been a pioneer in the development of novel Bayesian statistical methods to evaluate risk in vulnerable populations, particularly sickle cell patients. These methods will be used to identify variables that contribute to risk or protection in obese patient cohorts, as will be evaluated in Aim 1. Dr Denis has been a pioneer in the development of new flow cytometry-based multiparametric analysis of human leukocytes that infiltrate and inflame adipose tissues (obtained by bariatric surgery). New multicolor immunophenotyping protocols that avoid unappreciated the intrinsic autofluorescence of adipose tissue macrophages, for example, will require technology development and cytometry laser optimization as will be conducted in Aim 2. We will develop 6–8+ color cytometry; only 2-color cytometry has been reported, and much of what has been published has been poorly done, with many problems in fluorochrome compensation.
Meetings. It is anticipated that the ARC will meet every 8 weeks for progress reporting and to plan grant applications, including the coordination required to obtain preliminary data for an R21 application anticipated in 2011, and R01 to build on the expected results. Investigators for each Aim will be asked to keep the group informed of their progress at each meeting. The meetings will typically take place in the early evenings for 2 hours and will include refreshments and cheese to facilitate informal discussion and brain-storming. Given the importance of thinking outside of the home disciplines of the individual investigators, more meeting time will be allocated to discussion than to specific scientific presentations. Meetings will thus serve primarily to provoke additional discussion and insight, rather than to confirm straightforward progress.
References cited:
1. Zimmet, P., Alberti K.G. and Shaw J. (2001). Global and societal implications of the diabetes epidemic. Nature 414, 782 – 787.
2. Seidell, J.C. (2000). Obesity, insulin resistance and diabetes–a worldwide epidemic. Br. J. Nutr. 83 Suppl 1, S5 – 8.
3. Wild S, Roglic G, Green A, Sicree R, King H. (2004). Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047 – 1053.
4. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, et al. (2006). Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 17: 4 – 12.
5. Shoelson, S. E., Lee, J. & Goldfine, A. B. (2006). Inflammation and insulin resistance. J. Clin. Invest. 116, 1793 – 1801.
6. Kahn SE, Zinman B, Haffner SM, O’Neill MC, et al. (2006). Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes 55: 2357 – 2364.
7. Hotamisligil GS, Shargill NS and Spiegelman BM. (1993). Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259: 87 – 91.
8. Kim CS, Park HS, Kawada T, Kim JH, Lim D and Hubbard NE et al.. (2006). Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. (Lond) 30: 1347–1355.
9. Ruderman NB, Schneider SH and Berchtold P. (1981). The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 34: 1617 – 1621.
10. Bonora E, Willeit J, Kiechl S, Oberhollenzer F, Egger G, Bonadonna R, Muggeo M. (1991). U-shaped and J-shaped relationships between serum insulin and coronary heart disease in the general population. The Bruneck Study. Diabetes Care 21: 221 – 230.
11. Wildman RP, Muntner P, Reynolds K, et al. (2008). The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch. Intern. Med. 168: 1617 – 1624.
12. Succurro E, Marini MA, Frontoni S, Hribal ML, Andreozzi F, Lauro R, Perticone F, Sesti G. (2008). Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals Obesity (Silver Spring) 16, 1881 – 1886.
13. Wang F, Liu H, Blanton WP, Belkina A, LeBrasseur NK and Denis GV. (2009). Brd2 disruption in mice causes severe obesity without type 2 diabetes. Biochem J. 425: 71 – 83. PMID: 19883376
14. Belkina AC and Denis GV. (2010). Obesity genes and insulin resistance. Curr. Opin. Endocrinol. Diabetes Obes. 17: 472 – 477. PMID: 20585247
15. Woodard J, Joshi S, Viollet B, Hay N and Platanias LC. (2010). AMPK as a therapeutic target in renal cell carcinoma. Cancer Biol. Ther. 10. E-pub ahead of print.
16. Woodard J and Platanias LC. (2010). AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem. Biophys. Res. Commun. 398:135 – 139.
17. Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. (2006). The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 116: 1913 – 1923.
Pictures, Images, Figures:
********************************