Obesity: What Does Immunity Got to Do With it?
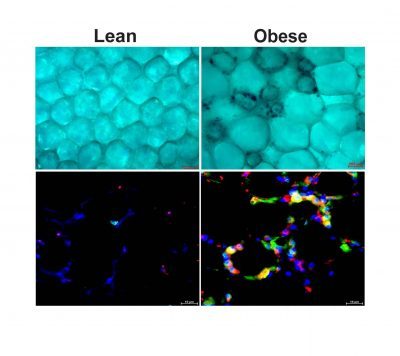 The top panel is an image of adipose tissue from lean and obese models where senescent macrophages appear in dark blue. The bottom panel is an image of adipose tissue from lean and obese models where macrophages are in green, a senescence marker (p21) is in red, the overlap in yellow represent senescent macrophages. In bleu is the cell nucleus
The top panel is an image of adipose tissue from lean and obese models where senescent macrophages appear in dark blue. The bottom panel is an image of adipose tissue from lean and obese models where macrophages are in green, a senescence marker (p21) is in red, the overlap in yellow represent senescent macrophages. In bleu is the cell nucleus
As organisms grow, older cells can undergo a phenomenon called senescence. This process defines a cell state where cells permanently stop dividing but do not die. Senescent cells secrete toxic pro-inflammatory factors contributing to the development of many diseases.
BUSM researchers have shown that obesity in experimental models led to senescence of macrophages, an immune cell subtype within fat or adipose tissue.
According to the researchers, the fact that macrophages can become senescent is an unexpected finding. Many of the macrophages within obese tissue were senescent and those senescent cells may be a significant driver of fat tissue fibrosis. These findings suggest that obesity accelerates cellular or biological immune aging in fat.
“In healthy individuals, those cells contribute to cleaning the tissue from dead adipocytes (cells specialized for the storage of fat) and help in the cellular turnover. We demonstrated that macrophages lost this capacity when they become senescent,” explained first and co-corresponding author Nabil Rabhi, PhD, an instructor of biochemistry at BUSM.
The researchers also found that senescent macrophages secrete a variety of factors, one of which is a molecule called osteopontin which they found is responsible for adipose tissue fibrosis. “Our finding suggests that macrophages ages faster in obese animals. This accelerated senescence may contribute to the pathological thickness or fibrosis of fat tissue observed in obese individuals with type 2 diabetes,” said Rabhi.
The researchers believe understanding new regulatory pathways that control adipose tissue responses to obesity may help identify new targets for obesity treatment. “Our finding indicates that targeting the senescent macrophages population or using osteopontin inhibition may represent a promising approach for obesity treatment and its adverse complication including type 2 diabetes,” added Rabhi.
Collaborators from BUSM included co-corresponding authors Matthew D. Layne, PhD, associate professor of biochemistry and assistant dean for research and Stephen R. Farmer, PhD, professor of biochemistry.
These findings appear online in the journal Life Science Alliance.
View all posts