Researchers Develop a Model of the Yeast Nuclear Pore Complex
Nuclear pore complexes (NPCs) are massive multi-protein complexes that act as passageways for the transport of molecules into and out of the nucleus. Given their central role in gene expression, growth and development, it is not surprising that NPC defects are linked to many diseases such as viral infections, cancers and certain neurodegenerative diseases, and that nuclear transport is a target for possible therapeutics.
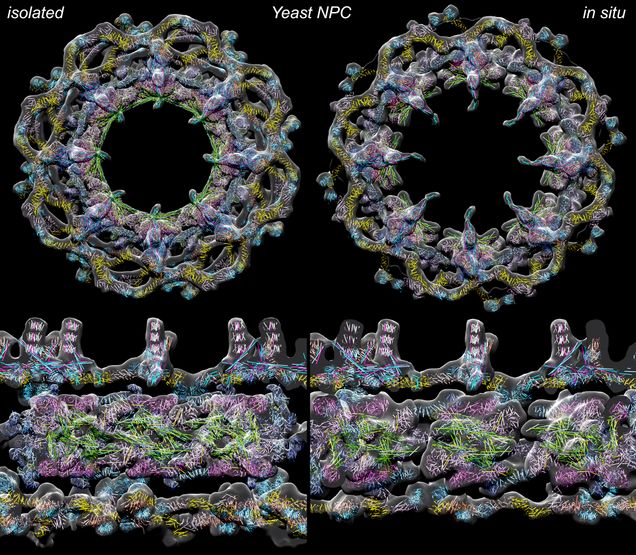 Two configurations of the yeast NPC are shown (left: contracted, right: expanded)
Two configurations of the yeast NPC are shown (left: contracted, right: expanded)
in top (upper) and side (lower) views with the latter at a larger scale.
Using rapid plunge freezing and cryo-EM (electron microscopy) with computational methods, BUSM researchers have produced a comprehensive model of the yeast NPC which reveals the interconnected architecture of its core scaffold. This work provides molecular models for two configurations: one that is easier to study in isolated samples to provide a more detailed overview of a radially-compact form and a second expanded form in the living yeast cell, albeit this “in situ” form is currently visualized with a lower level of detail.
“This research significantly extends our understanding of the architecture of the NPC from brewers’ yeast, a model organism that is used to study the biology of cells that contain a nucleus and thus provides new insights on multiple levels into the functions of this transport machine,” explains corresponding author Christopher W. Akey, PhD, professor of physiology and biophysics.
According to researchers, this model will provide a better understanding of how these large mega-channels assemble and how they can flex and adapt to changes in transport by expansion of their central passageway. “Moreover, we have observed multiple types of NPC in the same cell for the first time, which reflects the lego-like ability of this assembly to use interchangeable parts to modify its architecture on the nuclear side. This adaptability may play a role in tailoring the functions of these machines for different local environments at the periphery of the nucleus,” says Akey.
The researchers believe that these findings now set the table for studies of how viruses may usurp this important pathway to infect cells and alter their physiology to cause disease.
Collaborators on this project include Michael Rout, PhD, and Javier Fernandez-Martinez, PhD, from the Rockefeller University; Steve Ludtke, PhD, from Baylor College of Medicine and Elizabeth Villa, PhD, from the University of California, San Diego.
These finding appear online in the journal Cell.
View all posts