Cracking the NIH Code
Amid science funding’s grim realities, one group is making it work
Andrew Wilson read the e-mail. He took a few deep breaths. He read it again. It was a couple of days earlier than expected, but there it was, sitting in his inbox. Four months earlier, Wilson, a School of Medicine assistant professor of medicine, had submitted a proposal for a highly competitive R01 grant from the National Institutes of Health (NIH) to study the genetic disease alpha-1 antitrypsin deficiency. The disease, called alpha-1 for short, causes emphysema in the lungs and cirrhosis in the liver, and has been the focus of Wilson and his mentor, Darrell Kotton, for more than a decade. “We know the mutation responsible for the disease, and we’re powerless to do anything about it,” says Kotton. “On paper you can actually write down all the steps it would take to cure the disease. Not to treat it, but to cure it.”
Over the past months, a team of scientists had reviewed Wilson’s proposal for the NIH and scored its scientific merit and likelihood of success. The score waiting in Wilson’s inbox would tell him if the NIH would fund his work for the next five years, bringing science that much closer to a cure. Heady stuff, but there was also something personal at stake for Wilson. Although he had earned grant money before, winning an R01 would mean that he would finally become, as Kotton says, “a card-carrying, bona fide independent researcher.” He took another deep breath, then he went to the NIH website for his score.
“It’s actually one of the most tense, defining moments in a scientific career,” says Kotton, a founding director of BU’s Center for Regenerative Medicine(CReM), a MED professor of medicine and pathology, and an attending physician in pulmonary and critical care medicine at Boston Medical Center. “There are very few crystal clear moments in science, but that’s one of them. You know when you click on that button that you’re either going to see a score in a percentile that makes you happy, or you’re going to see the dreaded ND—not discussed.” ND means your grant has been “triaged,” sent packing without discussion and formal comments. “That’s hard to recover from, statistically,” says Kotton. “You really want a good score.”
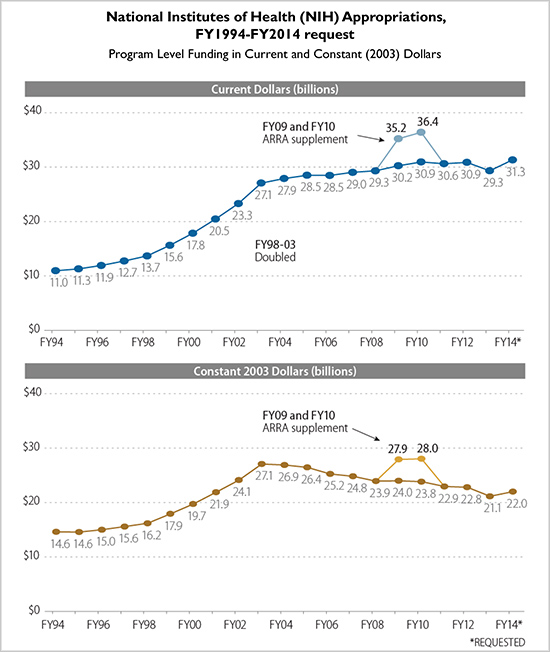
These are tough times to be a scientist relying on NIH money. The NIH is the major source of federal funding for biomedical research in the United States, supporting over 300,000 scientists working at more than 2,500 universities, hospitals, and research institutions, according to a report from the Congressional Research Service. Although researchers find money elsewhere—scientists in Kotton’s lab have secured funds from theMassachusetts Life Sciences Center, theAmerican Lung Association, and theAlpha-1 Foundation, among others—the NIH is the big enchilada, distributing about $30 billion in FY 2014. But that $30 billion figure has barely risen since 2003, and has actually declined in constant dollars. Researchers use words like “frustrating,” “grim,” and “painful” to describe the current funding situation. “The resulting strains have diminished the attraction of our profession for many scientists—novice and experienced alike,” notes a gloomy 2014 article in the journal PNAS, coauthored by formerNational Cancer Institute leader Harold Varmus. “Even the most promising trainees are increasingly pessimistic about the future of their chosen career.”
Except, it seems, for Darrell Kotton and his posse of scientists. Like a cluster of hardy cacti, they have managed to bloom in the desert. Kotton runs CReM with cofounders and codirectors Gustavo Mostoslavsky and George Murphy, both MED assistant professors of medicine, and their center is home to 34 scientists at multiple levels, from students to postdocs to faculty. Within CReM, Kotton, Wilson, and another scientist, Laertis Ikonomou, a MED assistant professor of medicine and a College of Engineering assistant professor of biomedical engineering, have created an unusual three-headed lab group they informally call KIWI, for Kotton, Ikonomou, and Wilson. “We always worked closely together, and just decided it was a good model,” says Ikonomou, who trained under Kotton. “It’s much more efficient.” For instance, he says, if one of Kotton’s technicians finds a new recipe for growing stem cells, he or she shares it with the group. “Then I don’t have to make the recipe from scratch,” he says. “It saves a lot of time and effort.”
The rainmaker
All three scientists are principal investigators—PIs in science lingo—who raise their own money, but they hold joint lab meetings, share ideas and equipment, and pool all their grants and resources. “That way the science isn’t so disproportionately influenced by the waxing and waning funding of any individual PI,” says Kotton. At the moment, though, and for the foreseeable future, the K of KIWI is the group’s biggest rainmaker. Since fiscal year 2005, Kotton alone has received $9,692,409 in direct NIH funding, as well as $406,550 in NIH “flow-through” funding—money given to other researchers who subcontract to Kotton and his scientists. That’s in addition to the millions he’s pulled in from foundations and other sources. No wonder he seems perpetually upbeat. “I think we’re the happiest guys in the world,” he says often—and means it. Visitors to his lab wonder what he’s been drinking and where they might get some.
“He’s just a glass-half-full kind of person,” says Linda Hyman, associate provost for the Division of Graduate Medical Sciences at MED. “I’m sure he has his moments, but his upbeat nature is infectious and people want to work with him.” When Hyman needed someone to speak to potential graduate students thinking of coming to BU, Kotton was the obvious choice. “We wanted to put our best face forward, and he was just awesome,” says Hyman. “I think half the people who came to BU came because of Darrell’s talk.”
His optimism spills over to the younger scientists in his lab. “I think whining is harmful,” says Kotton. “We’re really trying to protect the young—meaning our graduate students and younger scientists—from a kind of negative thinking. We have to train them in the scientific method and put them in the most fertile soil so they can thrive. That isn’t a soil contaminated with a lot of whining.”
Finn Hawkins, a MED assistant professor of medicine and a junior member of Team KIWI, is a case in point. A physician, Hawkins came to Boston for a pulmonology fellowship in Kotton’s lab. But he was so inspired by Kotton and captivated by the science that he never left. “My mind was totally blown,” he says. “I had no idea what I had been missing.” Still, the choice of science as a career sometimes gives him pause. “I’ve seen some talented researchers really struggle,” he says. “But I’m in the best possible situation—I have a great mentor, there’s good science, and I’m working as hard as I can. When you’re a young researcher, there’s so much gloom and doom around. You have to stay optimistic.”
“I think whining is harmful,” says Kotton. “We’re really trying to protect the young—meaning our graduate students and younger scientists—from a kind of negative thinking.”
Of course, optimism alone doesn’t bring in the grants. Kotton’s team is full of gifted scientists, working in two fields—stem cells and regenerative medicine—that Hyman describes as very sexy. The KIWI group combines physician-scientists and straight PhDs, and Kotton says this mixture helps them envision clinical applications for their work—the bench-to-bedside payoff that many funders now demand. This is evident is Kotton’s overall research goal: to cure inherited lung diseases like alpha-1 and cystic fibrosis using induced pluripotent stem (iPS) cells—adult skin cells that scientists reprogram, giving them the ability to grow into almost any type of tissue. In the near term, iPS cells may serve as surrogates for a patient, allowing doctors and scientists to test a battery of drugs on a patient’s cells before trying them on the actual patient. That’s the promise of personalized medicine, another field high on the “very sexy” scale.
Within Kotton’s KIWI crew, Ikonomou focuses on the biochemistry of the primordial progenitors, the 100 or so undifferentiated stem cells that eventually lead to all lung cells. Wilson works from a disease angle, studying how stem cells from patients with alpha-1 grow into abnormal liver cells, while those from healthy people develop normally. That, broadly, was the subject of his NIH grant proposal, the one with the score still sitting in his inbox.
An enormous capacity for failure
Wilson clicked on the NIH website for his score: it was an 11. That means his grant ranked in the top 11th percentile. But in today’s dismal funding environment, even an outstanding score like 11 is no guarantee of funding. Scientists have to hit the payline, the cold cutoff above which no money will be offered. These days, around 10 percent of NIH grant proposals are funded, says William Cruikshank, director of the MED Graduate Program in Molecular and Translational Medicine and a MED professor of medicine, pathology, and laboratory medicine. In the heady days of high funding, that number approached 20 percent; the lowest he’s seen has been 5 to 6 percent. “The economy went down and NIH funding went down with it,” Cruikshank says. At the same time, the cost of biomedical research, from fancy freezers to mouse models, has increased, leaving many scientists queasy over funding. “The real frustration is that you put in one grant and it’s bare-bones—just what you need to accomplish your minimum goals and nothing else,” he says. “Then that may get cut programmatically, and your bare-bones budget gets even smaller.” The result is that most scientists cannot survive on one large NIH grant, as was traditionally the case. Instead, it’s a constant scramble to write the papers and generate the data to create more strong proposals asking for yet more money. “It’s truly a rat race to survive,” he says.

That’s why Kotton’s system of pooling resources is so beneficial, Cruikshank says. But there’s another, critical benefit: the collaboration improves the science. “Kotton has assembled a team with a lot of different expertise, and they fit together like a jigsaw puzzle,” he says. “Everybody’s coming at the same problem from different directions. This lets you ask the broad questions that make science exciting. If everyone’s inbred, it doesn’t really go anywhere.”
“We want to collaborate with as many people as we can. The constant, free exchange of ideas really helps the science,” says Ikonomou. “Being well-funded is just a side benefit.”
Kotton also has the rare gift of knowing how to explain the science. “He can communicate the excitement and relevance of what he does to scientists, the public, and investors,” says Hyman. “It’s a talent. He can tell the story.”
It’s a talent that’s critical for grant writing, and one that Kotton takes pains to pass on to younger scientists. He had carefully coached Wilson through the long process of writing, and rewriting, his NIH proposal. Wilson had asked the NIH for $1.25 million over five years—the standard R01 award—to study alpha 1 in liver cells. Here’s how he explains the proposal: “We think the cells are surrogates for the patient. That’s widely believed to be the case, but it hasn’t actually been proven. So for this grant we teamed up with a clinical trial in Pittsburgh of patients with alpha-1 and liver disease. There’s a seizure medicine that has a side effect, which happens to help the liver cells deal with the abnormal alpha-1 antitrypsin protein inside them. It works in mouse models, and so in Pittsburgh they’re giving these patients this drug for a year, and they’re doing liver biopsies before and after. So we’re making iPS cells from the patients who are in the trial, and basically trying to run the trial in a dish.”
Kotten nods at his protégé, smiling at the perfectly executed pitch. “It’s good stuff, isn’t it? It totally makes sense.”
“One of the hallmarks of people who are successful in science these days is a willingness to persist in the face of failure and rejection,” says Wilson.
It is good stuff. The NIH agreed. When Wilson saw his score was in the 11th percentile, he knew it was good. But was it good enough to hit the payline? He took a screenshot of the website and e-mailed it to Kotton. “I thought I knew what it meant,” he says, “but I just needed someone else to look at it and confirm what I was seeing, so I could believe it was real.” Kotton called him moments later—that year’s payline was 13. The grant would be funded.
And if it hadn’t been? “One of the hallmarks of people who are successful in science these days is a willingness to persist in the face of failure and rejection,” says Wilson. “And so I would have kept trying.”
The feeling is echoed by younger Kotton mentee Hawkins, who says his biggest strength is not creativity or intelligence, but “an enormous capacity for failure.”
“Maybe if we actually paused and thought about the statistics for a moment, we’d realize we’re crazy and stop doing science,” says Kotton. “But we’re getting paid to be children. We play in the science lab, we do experiments, we try to figure out how nature works. I work with really smart young people, and mentor them, and really enjoy their success and emergence. It’s really an amazing career. I wouldn’t trade it for anything.”
This BU Today story was written by Barbara Moran. She can be reached at bmoran@bu.edu.