Research
 We continually strive to ensure our laboratory remains a vibrant, fun, and rigorous environment where trainees from across the world come for mentorship and the pursuit of new knowledge. We are most proud of the graduates of our lab, including PhD graduates, pulmonary MD fellows and post-doctoral PhD fellows, many of whom have gone on to independent careers as basic science researchers. Truth, scholarship, rigor, curiosity, and ‘Open Source Biology’ sharing continue to be the underpinnings of our training style.
We continually strive to ensure our laboratory remains a vibrant, fun, and rigorous environment where trainees from across the world come for mentorship and the pursuit of new knowledge. We are most proud of the graduates of our lab, including PhD graduates, pulmonary MD fellows and post-doctoral PhD fellows, many of whom have gone on to independent careers as basic science researchers. Truth, scholarship, rigor, curiosity, and ‘Open Source Biology’ sharing continue to be the underpinnings of our training style.
An overview of active lab projects is summarized below. We invite those interested in more detailed views of our work to click on the following link to our most recent publications on pub med.
Project Areas Under Study, By Theme:
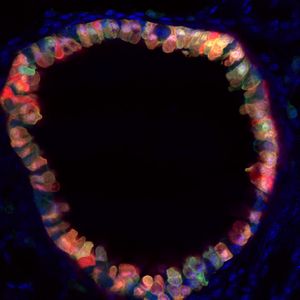
Project Theme 1: De novo derivation of lung cell lineages from pluripotent stem cells
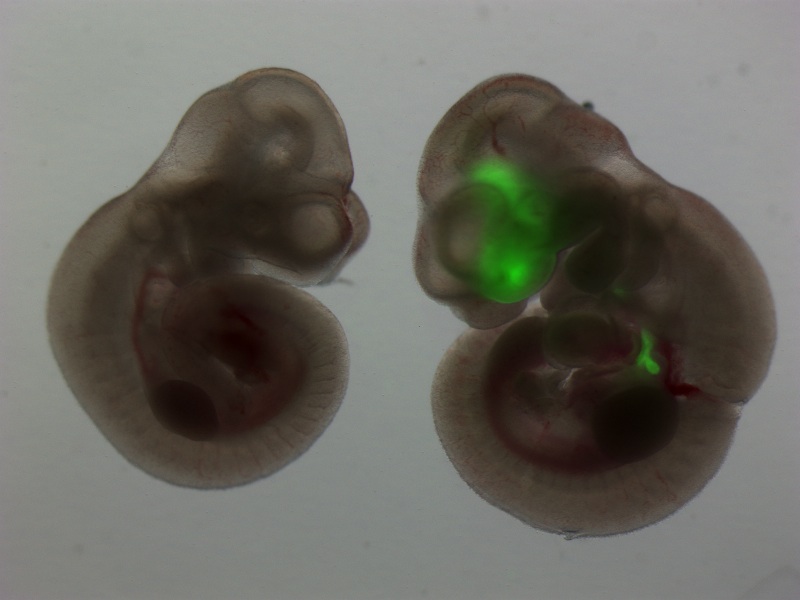 Lung disease is responsible for tremendous worldwide suffering. Our lab is interested in developing new treatments for genetic lung diseases, such as the two most common inherited lung diseases: emphysema due to alpha-1 antitrypsin deficiency and cystic fibrosis. We also develop reconstituting therapies designed to replenish the injured or degenerating lung epithelium, such as that seen in tobacco smoke-induced COPD/emphysema, idiopathic pulmonary fibrosis, ARDS, cystic fibrosis, and a variety of childhood genetic lung diseases.
Lung disease is responsible for tremendous worldwide suffering. Our lab is interested in developing new treatments for genetic lung diseases, such as the two most common inherited lung diseases: emphysema due to alpha-1 antitrypsin deficiency and cystic fibrosis. We also develop reconstituting therapies designed to replenish the injured or degenerating lung epithelium, such as that seen in tobacco smoke-induced COPD/emphysema, idiopathic pulmonary fibrosis, ARDS, cystic fibrosis, and a variety of childhood genetic lung diseases.
A unifying approach we have taken over the past decade is the de novo derivation of lung lineages from pluripotent stem cells. We have published tools and approaches for the derivation and purification of primordial lung progenitors based on cell sorting endodermal cells expressing the transcriptional regulator, Nkx2.1. This locus is the first to be activated during the cell fate decision to become either lung epithelium or thyroid epithelium, which occurs early in embryonic development within the anterior foregut’s developing endoderm. Since the entire lung epithelium develops from these rare Nkx2.1+ primordial lung progenitors, our lab is particularly focused on understanding the regulation of the Nkx2.1 gene locus, including its epigenetic status. A rare cohort of children with mutations in this locus, suffer from the Brain-Lung-Thyroid syndrome and hence this is one of the disease targets of our work, which is performed in collaboration with the Children’s Interstitial Lung Disease Foundation (chILD).
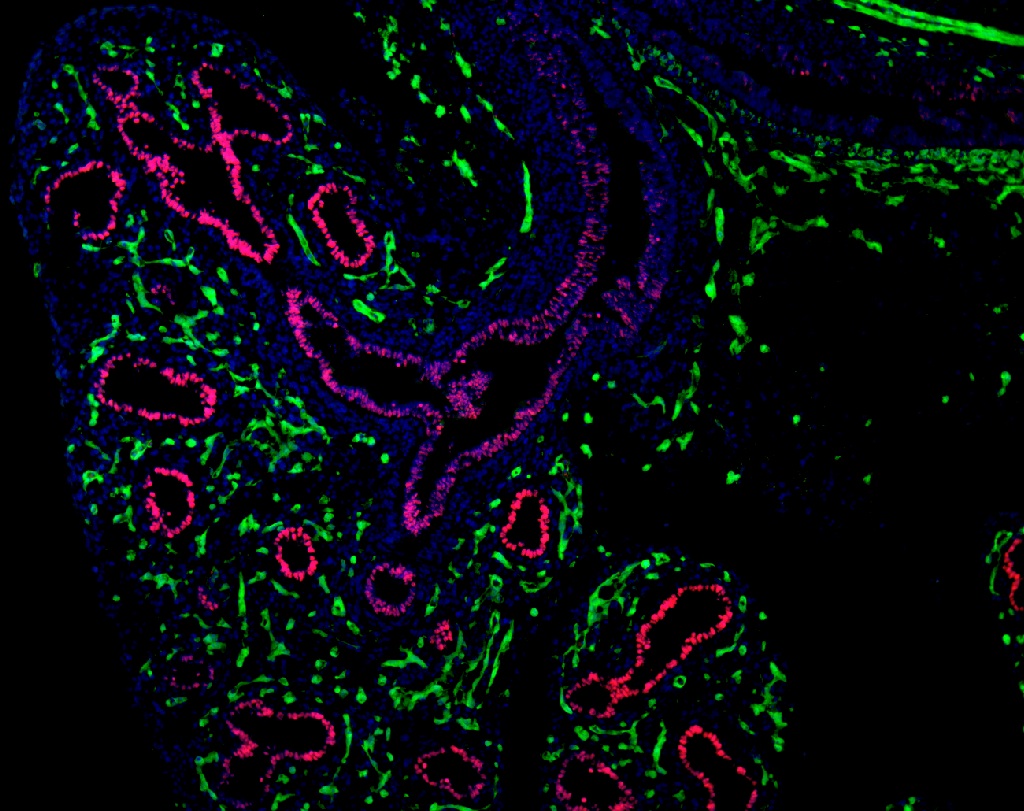
Beyond the lung epithelium, lung structure also includes vasculature and interstitium. We have active projects in our lab using pluripotent stem cells to also derive lung endothelium, fibroblasts, and smooth muscle cells as part of our long-term goal of generating the full cellular diversity and structural complexity of the entire lung organ for future transplantation studies and disease models.
Project theme 2: New therapies for lung diseases, based on increased understanding and stem cell models.
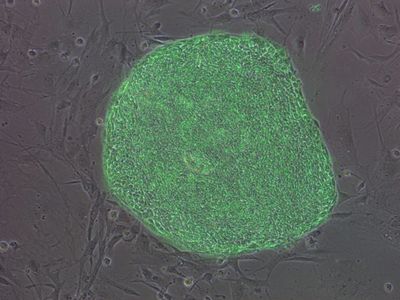
 In previous years we have developed technologies for reprogramming patient skin or blood specimens into iPS cells. We have generated a large bank of lung disease-specific iPS cells and we are currently focused on using this bank to model a variety of lung diseases. These in vitro disease models allow a better understanding of disease pathogenesis and enable the development of novel gene therapies, cell-based reconstituting therapies, as well as optimized screens of new therapeutic drugs. We believe patient-specific iPS cells provide a platform for the ultimate accomplishment of personalized medicine, the tailoring of therapies individualized for each patient’s own genetic background and unique disease phenotype. In the past we have published a proof-of-principle of this concept, generating iPS cells to understand individualized drug therapy for a patient suffering from long QT syndrome. In the future we hope to apply this approach to better understand and treat the following diseases:
In previous years we have developed technologies for reprogramming patient skin or blood specimens into iPS cells. We have generated a large bank of lung disease-specific iPS cells and we are currently focused on using this bank to model a variety of lung diseases. These in vitro disease models allow a better understanding of disease pathogenesis and enable the development of novel gene therapies, cell-based reconstituting therapies, as well as optimized screens of new therapeutic drugs. We believe patient-specific iPS cells provide a platform for the ultimate accomplishment of personalized medicine, the tailoring of therapies individualized for each patient’s own genetic background and unique disease phenotype. In the past we have published a proof-of-principle of this concept, generating iPS cells to understand individualized drug therapy for a patient suffering from long QT syndrome. In the future we hope to apply this approach to better understand and treat the following diseases:
- COPD/emphysema
- Alpha 1 antitrypsin deficiency
- Cystic Fibrosis
- Childhood Interstitial Lung Diseases (ChILD, including those due to genetic mutations in the lung epithelial genes, NKX2.1, SPC, SPB, and ABCA3.)
- Inherited Pulmonary Vascular Diseases due to BMPR2 mutations
- ARDS
We are supported by several patient-focused foundations to target the above diseases, including The Alpha-1 Foundation, The Cystic Fibrosis Foundation, the Americal Lung Association, and the ChILD Foundation. We have a particularly long and fruitful relationship with the Alpha-1 Foundation as our investigators serve on the Foundation’s scientific boards and Grants Advisory Council. For many years this remarkable Foundation has funded our gene therapy and stem cell work whose focus is understanding and better treating both the lung and liver diseases, caused by Alpha-1. In 2012, Drs. Andrew Wilson and Darrell Kotton co-founded BU/Boston Medical Center’s “The Alpha-1 Center”, dedicated to the comprehensive care of patients and families suffering from Alpha-1. In recognition of our research contributions, in 2010 Dr. Kotton received the Alpha-1 Foundation’s ‘Golden Shillelagh Award’, given each year to honor a researcher whose work most impacts those with alpha-1 antitrypsin deficiency. In 2013, Dr. Kotton was awarded the Foundation’s ‘Alpha-1 Researcher of the Year’ prize.
Project theme 3: Developmental biology of the lung
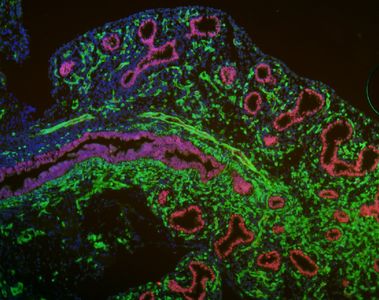 The lung is a beautiful organ. Its embryonic development is complex and barely understood. More than just a passion and curiosity of ours, understanding lung development holds the key for developing stem cell therapies for our patients. Since our ES cells and iPS cells resemble the earliest, pluripotent state of the developing embryo, consequently we believe the most efficient way to derive lung cells from these pluripotent stem cells is to recapitulate in vitro the full sequence of developmental milestones that Nature employs in vivo each time an embryo forms lungs during pregnancy. It is known that the lung develops from the definitive endoderm germ layer in the embryo, and hence we have a long history of publications studying the basic biology of definitive endoderm development as well as the development of downstream endodermally-derived lineages such as the lung, thyroid, liver, pancreas, and intestine. An important focus of our lab remains studying and understanding the basic biology of endodermal and lung developmental biology, using in vivo mouse models as well as in vitro mouse and human ES cell and iPS cell models.
The lung is a beautiful organ. Its embryonic development is complex and barely understood. More than just a passion and curiosity of ours, understanding lung development holds the key for developing stem cell therapies for our patients. Since our ES cells and iPS cells resemble the earliest, pluripotent state of the developing embryo, consequently we believe the most efficient way to derive lung cells from these pluripotent stem cells is to recapitulate in vitro the full sequence of developmental milestones that Nature employs in vivo each time an embryo forms lungs during pregnancy. It is known that the lung develops from the definitive endoderm germ layer in the embryo, and hence we have a long history of publications studying the basic biology of definitive endoderm development as well as the development of downstream endodermally-derived lineages such as the lung, thyroid, liver, pancreas, and intestine. An important focus of our lab remains studying and understanding the basic biology of endodermal and lung developmental biology, using in vivo mouse models as well as in vitro mouse and human ES cell and iPS cell models.
Project theme 4: Gene therapy of lung disease
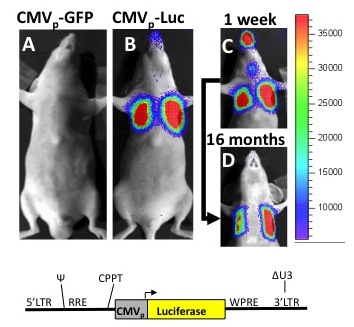
We have a long history of developing lentiviral and other vector based approaches for the future treatment or cure of genetic lung diseases, such as alpha-1antitrypsin deficiency. New gene therapy approaches, involving in vivo lentiviral instillations in mice or gene editing of pluripotent stem cells in vitro continue to be an important focus of our lab and follow a history of publications in these areas.