Joel M. Henderson MD, PhD
Associate Professor
Contact Information
Department of Pathology & Laboratory Medicine
Boston University School of Medicine 670 Albany Street, Rm 347 Boston MA, 02118
617-414-7063 (phone)
617-414-5315 (fax)
jmhender@bu.edu
Education
- BS (Engineering Mechanics), University of Cincinnati
- MD, PhD (Biomedical Engineering), Ohio State University
- Resident in Anatomic Pathology, Brigham and Women’s Hospital
- Clinical Fellow in Renal Pathology, Brigham and Women’s Hospital
- Research Fellow in Pathology/Nephrology, Brigham and Women’s Hospital
Research Topic: Role of the Podocyte in Mechanically-Mediated Glomerular Damage
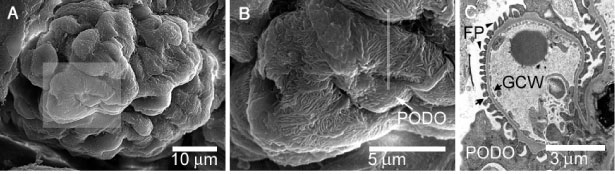
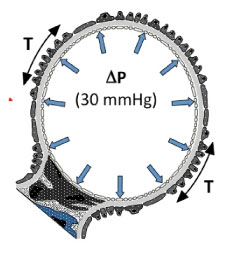
Glomeruli are a sub-millimeter-sized corpuscles of intertwining capillaries that are found in the kidney. About 500,000 glomeruli are present in each human kidney. In the glomeruli, waste products are filtered from the blood as the first step in the formation of urine. The blood ultrafiltrate that eventually becomes urine is forced through the glomerular capillary walls under high pressure. Podocytes, the structurally intricate epithelial cells that surround the outside of the glomerular capillaries, play an important role in forming and regulating this structurally complex permability barrier (Figure 1). Podocytes are also likely to play a key role in providing structural strength to the glomerular capillary wall, so that it is able to resist the tension forces that develop in the wall due to the blood pressure gradient that drives ultrafiltration (Figure 2).
Our overriding hypothesis is that progressive glomerular damage can occur when: (1) glomerular capillary blood pressures are increased beyond their normally high levels, or (2) podocytes are structurally compromised in some way that limits their ability to resist normal capillary blood pressures. The resulting podocyte injury is initially only visible under the electron microscope, but is ultimately manifest as irreversible glomerular scarring and senescence, known as glomerulosclerosis. This sequence is the “final common pathway” of a large number of kidney diseases, and eventually leads to chronic renal failure and end stage kidney disease (ESKD), requiring dialysis or kidney transplantation to replace the function of damaged kidneys.
Our laboratory studies the role of mechanical phenomena in glomerular injury, focusing on the podocyte. Specifically, we are defining the role of blood pressure forces within the glomerular capillary in causing podocyte injury and progression to glomerulosclerosis. Our goals are to: (1) determine the structural features of the podocyte that are responsible for the mechanical behavior of these cells and the mechanical properties of the glomerulus; (2) determine how elevated intracapillary pressures are translated into changes in glomerular capillary wall mechanics that are associated with mechanically-mediated damage; (3) characterize the effect of altered capillary wall mechanics on normal podocyte function (e.g., glomerular filtration, protein synthesis, etc.); (4) identify the mechanisms by which elevated intracapillary pressures lead to podocyte injury and glomerulosclerosis; and (5) determine how known disease-causing genetic mutations can leave the podocyte more vulnerable to mechanically-mediated damage. Ultimately, progress in achieving these goals may lead to treatments that can slow or halt the progressive kidney damage that develops subsequent to many kidney diseases.
An important characteristic of our research program is its multidisciplinary nature. We welcome individuals with backgrounds in medicine, pathology, biological and physical sciences, and engineering. In fact, much of our effort is directed toward pioneering new approaches for observing and measuring physical phenomena in biological tissues at the micro- and nano- scales. These measurements are complex, due to the small size of these structures and the challenge of physically accessing these tissues while maintaining them in a functional state. The need for new tools that will facilitate these complex measurements presents an ideal opportunity for engineers and physical scientists with an interest in biomedical research. We employ a wide spectrum of methodologies from the physical and biological sciences in pursuit of our research goals, including the full complement of cell and molecular biologic techniques, transgenic animal models, a wide variety of microscopic imaging techniques (traction force microscopy, multi-photon fluorescence microscopy, scanning and transmission electron microscopy, etc.), and microfabrication. We are also fortunate to be located in Boston, which makes it relatively easy to collaborate with many of the leaders in kidney disease and biophysics research.
Current Projects
Biomechanics of the Podocyte – Traction Force Microscopy to Measure Podocyte Contractility
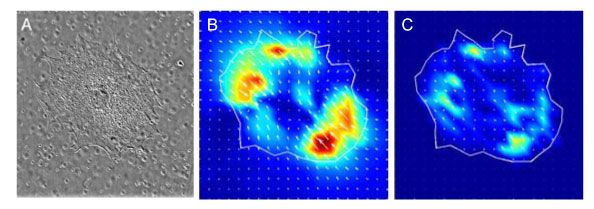
Traction is the tension or “pulling” force exerted by a cell on the anchoring surfaces to which it is attached. Such tension forces are integral to maintenance of tissue structure, and may be modulated in response to a wide variety of soluble and mechanical stimuli. In the glomerulus, tension forces exerted by the podocyte contribute to the stiffness of the glomerular capillary wall. In collaboration with the laboratory of Dr. Donald Ingber at Boston Children’s Hospital, we are using traction force microscopy (TFM) to study traction forces generated by cultured podocytes (Figure 3). We are coupling molecular techniques, including RNA interference, to selectively modulate expression of key proteins that are involved in generation of traction forces in podocytes, such as alpha-actinin-4 and non-muscle myosin IIA. In addition, we are very interested in studying podocyte traction in response to mechanical stimuli, and are actively developing new methods for doing this.
Animal Models of Kidney Disease Involving the Podocyte Contractile Cytoskeleton
We are studying mouse models of chronic glomerular disease in our laboratory. These are aimed toward extending our in vitro work and increasing our understanding of: (1) the pathology of kidney damage associated with alterations in disease-associated proteins, and (2) the role of these key structural proteins in resisting blood pressure forces in the glomerular capillaries.
Mouse models of Actn4 – In collaboration with the laboratory of Martin Pollak at Brigham and Women’s Hospital, we are studying the development of glomerular damage in mice carrying Actn4 mutations similar to those associated with human disease. We have previously shown that mice that are homozygous for mutations in Actn4, or mice that do not express any form of alpha-actinin-4 protein, develop severe kidney damage with features of collapsing glomerulopathy, the most severe form of podocyte injury. However, mice that are heterozygous for mutations in Actn4 show very mild ultrastructural changes, but never develop significant kidney damage. The presence of mild ultrastructural changes suggests that there are subtle structural abnormalities which could render the podocyte and glomerulus vulnerable to damage under conditions of stress. We are currently pursuing this possibility by studying heterozygous Actn4 mutant mice after surgical ablation of most renal mass (5/6 nephrectomy model), or after pharmacologic manipulation intended to stress the kidney.
Mouse models of Myh9 – We are developing a mouse model that will be used to study the role of non-muscle myosin IIA in the development of glomerulosclerosis. Non-muscle myosins are the motor proteins that are likely to be responsible for tensile force generation in most cells. Non-muscle myosin IIA, the protein product of the gene MYH9, is a ubiquitous myosin II isoform that is expressed in human and murine podocytes, and is known to be associated with human glomerular disease. Mutations in MYH9 are associated with a spectrum of diseases that are primarily associated with hematologic disorders, but that may also lead to kidney lesions manifest as FSGS. Further, the increased propensity for chronic kidney disease in African Americans has been recently shown to be strongly associated with genetic variation in the immediate vicinity of the MYH9 gene.
Biomechanics of the Glomerulus – Intravital Imaging as a Tool for Measuring Glomerular Mechanics
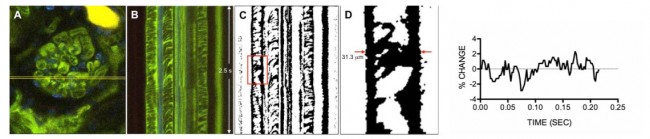
We are using intravital multi-photon microscopy to measure mechanical phenomena in the intact, living glomerulus, in order to fully characterize podocyte mechanical function and response. This work is being done in collaboration with the Center for Advanced Renal Microscopic Analysis at the Indiana University School of Medicine, directed by Bruce Molitoris, MD. Developing the capability to conduct these studies in the murine model will be particularly beneficial, as this will allow us to identify differences in the mechanical behavior of glomeruli in genetically manipulated animal models. The approach to these measurements is described in Figure 4. Our preliminary data suggests that there is normally little motion of the murine glomerular capillary wall across the cardiac cycle (approximately 4% maximum change in capillary width), despite an expected glomerular capillary pressure change of about 20% over the cardiac cycle, based upon micropuncture experiments performed by others (Aukland et al, Acta Physiol Scand 1977, 101:418-427). This suggests that the wild type murine glomerular capillary is a rigid structure; studies in animals such as the Actn4 and Myh9 murine models described above may allow us to detect differences in the structural rigidity of the glomerular capillaries that predispose to glomerular capillary damage.
Kidney Pathology in Human Glomerular Disease
Kidney structure and function in patients with genetic risk factors for chronic glomerular disease – We are currently studying human kidney tissue to identify structural and functional characteristics of kidneys from patients with mutations or other genetic risk factors for chronic kidney disease. We are focusing on those risk factors involving podocyte injury, such as mutations in ACTN4, or genetic variation in the immediate vicinity of the MYH9 gene.
Collaborative Projects in Kidney Pathology and Glomerular Disease Research
We collaborate with other researchers to understand lesions that develop in the kidneys or associated organs of their animal models. We have helped characterize kidney lesions in mouse models of multiple myeloma, diabetes, autoimmune disease, acute tubular injury, and polycystic kidney disease.
Kidney Pathology Service at Boston Medical Center
We provide kidney pathology services for Boston University Medical Center. In this role we interact with nephrologists and nephrology trainees, transplant surgeons, and pathology faculty and residents. Research projects frequently arise from these interactions.
Publications
Wyss HM, Henderson JM, Byfield FJ, Bruggeman LA, Ding Y, Huang C, Suh JH, Franke T, Mele E, Pollak MR, Miner JH, Janmey PA, Weitz DA, Miller RT. Biophysical properties of normal and diseased renal glomeruli. Am J Physiol Cell Physiol. 2011 300:C397-405.
Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Uscinski AL, Higgs HN, Henderson JM, Pollak MR. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2010; 42:72-6.
Henderson JM, Alexander MP, Pollak MR. Distinctive features of glomerular injury in patients with ACTN4 mutations. J Am Soc Nephrol. 2009; 20:961-8.
Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008; 9:405-14.
Henderson JM, al-Waheeb S, Weins A, Dandapani SV, Pollak MR. Mice with altered alpha-actinin-4 expression have distinct morphologic patterns of glomerular disease. Kidney Int. 2008; 73:741-50.
Henderson JM, Alexander MP, Pollak MR. Distinctive features of glomerular injury in patients with ACTN4 mutations. J Am Soc Nephrol. 2009; 20:961-8.
Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008; 9:405-14.
Henderson JM, al-Waheeb S, Weins A, Dandapani SV, Pollak MR. Mice with altered alpha-actinin-4 expression have distinct morphologic patterns of glomerular disease. Kidney Int. 2008; 73:741-50.
Pollak MR, Alexander MP, Henderson JM. A case of familial kidney disease. Clin J Am Soc Nephrol. 2007; 2:1367-74.
Mistry K, Ireland JH, Ng RC, Henderson JM, Pollak MR. Novel mutations in NPHP4 in a consanguineous family with histological findings of focal segmental glomerulosclerosis. Am J Kidney Dis. 2007; 50:855-64.
Pao LI, Kong-Peng L, Henderson J, Kutok J, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific Shp1 deletion promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007; 27:35-48.
Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, Henderson J, Kretzler M, Cohen C, del Re E, Erickson A, Kerjaschki D, Rudensky A, Nikolic B, Reiser J. Proteolytic processing of dynamin by cytoplasmic cathepsin L defines a mechanism for proteinuric kidney disease. J Clin Invest. 2007; 117:2095-104.
Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, Zheng M, Mani M, Henderson J, Pinkus GS, Munshi N, Horner J, Ivanova EV, Protopopov A, Anderson KC, Tonon G, DePinho RA. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007; 11:349-60.
Humphreys B, Vanguri V, Henderson J, Antin J. Minimal change nephrotic syndrome and acute renal failure after vaccination in a hematopoietic stem cell transplant recipient. Nat Clin Pract Nephrol. 2006; 2:535-9.
Habicht A, Clarkson MR, Yang J, Henderson J, Brinkmann V, Fernandes S, Jurewicz M, Yuan X, Sayegh MH. Novel insights into the mechanism of action of FTY720 in a transgenic model of allograft rejection: implications for therapy of chronic rejection. J Immunol. 2006; 176:36-42.
Tatli S, Yucel EK, Couper GS, Henderson JM, Colson YL. Aneurysm of an aberrant systemic artery to the lung. Am J Roentgen. 2005; 184(4):1241-4.
Humphreys BD, Sharman JP, Henderson JM, Clark JW, Marks PW, Rennke HG, Zhu AX, Magee CC. Gemcitabine-associated thrombotic microangiopathy. Cancer. 2004; 100(12):2664-70.
Yao J, Le TC, Kos CH, Henderson JM, Allen PG, Denker BM, Pollak MR. Alpha-actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS Biol. 2004; 2(6):787-94.
Taylor EN, Henderson JM, Rennke HG, Magee CC. Traumatic calcinosis cutis in a dialysis patient. Am J Kidney Dis. 2004; 44(2):e6-9.
Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, Sugimoto H, Kalluri R, Gerszten RE, Pollak MR. Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest. 2003; 111(11):1683-90.
Abstracts (selected)
Histopathologic changes elicited in baboon kidneys after challenge with Shiga toxin type 1 or 2 from enterohemorrhagic E. coli. Henderson J, Kurosawa S, Stearns-Kurosawa D. 2011 United States and Canadian Academy of Pathology Annual Meeting, San Antonio, TX.
Intravital measurement of glomerular capillary displacement in rodents. Henderson J, Rhodes G, Bondzie P, Lucien L, and Molitoris B. 2010 American Society of Nephrology Annual Meeting, Denver, CO.
Distinct contributions of non-muscle myosin heavy chains IIA (Myh9) and IIB (Myh10) to podocyte traction and adhesion. Henderson J, Chen H, Tomolonis J, Cao M, Pollak M. 2010 American Society of Nephrology Annual Meeting, Denver, CO.
Role of myosin II and angiotensin in podocyte contractility in vitro. Henderson J, Wei C, Cao M, Reiser J, Pollak M. 2007 American Society of Nephrology Annual Meeting, San Francisco, CA.
Weins A, Schlondorff J, Henderson J, Pollak M. FSGS-causing mutations in alpha-actinin-4 reveal a novel mechanism for regulating Its actin-binding affinity. 2007 American Society of Nephrology Annual Meeting, San Francisco, CA.
Alpha-actinin-4 deficient mice develop focal and segmental glomerulosclerosis with features of collapsing glomerulopathy. Al-Waheeb S, Henderson J, Weins A, Dandapani S, Pollak M. 2007 United States and Canadian Academy of Pathology 2007 Annual Meeting. Stowell-Orbison Award for best paper through the medium of a poster presentation awarded to S. Al-Waheeb.
Frequency and significance of histopathologic features of collapsing glomerulopathy in end stage kidney disease (ESKD). Bijol V, Mendez G, Henderson J, Nosé V, Rennke H . Lab Invest. 2006;86:259A.
Relevance of thrombotic microangiopathy in native nephrectomy specimens obtained at time of transplantation. Henderson J, Hegedus A, Lin J, Rennke H, Chandraker A. J Am Soc Nephrol. 2004;15:885A.