Towards a comprehensive model of the yeast NPC
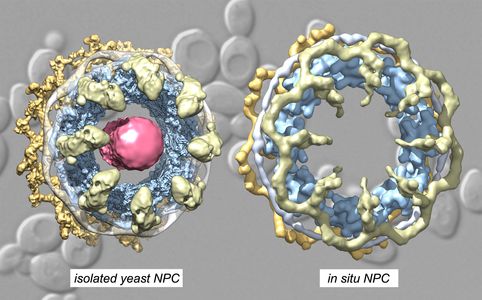
The nucleus is compartmentalized by double membranes of the Nuclear Envelope (NE) and macromolecules must cross this barrier during growth and differentiation. The Nuclear Pore Complex (NPC) forms a conduit for the bidirectional transport of proteins and RNA-protein complexes between the cytoplasm and nuclear interior. The large size, modular construction and dynamic nature of the 8-fold symmetric, yeast NPC (~600Å by 1100Å; 52-60 MDa ~550 nucleoporins/Nups) requires a multi-scale approach to obtain a complete model of this translocation machine, which spans large pores in the nuclear envelope. This work will inform us on the roles of different Nups in hierarchical assembly of the spoke-ring scaffold, pore membrane stabilization, spoke dynamics, structure and function of the central transporter and NPC evolution. We extended our analysis of the isolated NPC in an active collaboration with the Villa laboratory (UCSD) and are thus able to compare structures of contracted and radially expanded conformations in the isolated and in situ yeast pore complex.
Click on the figure for animation
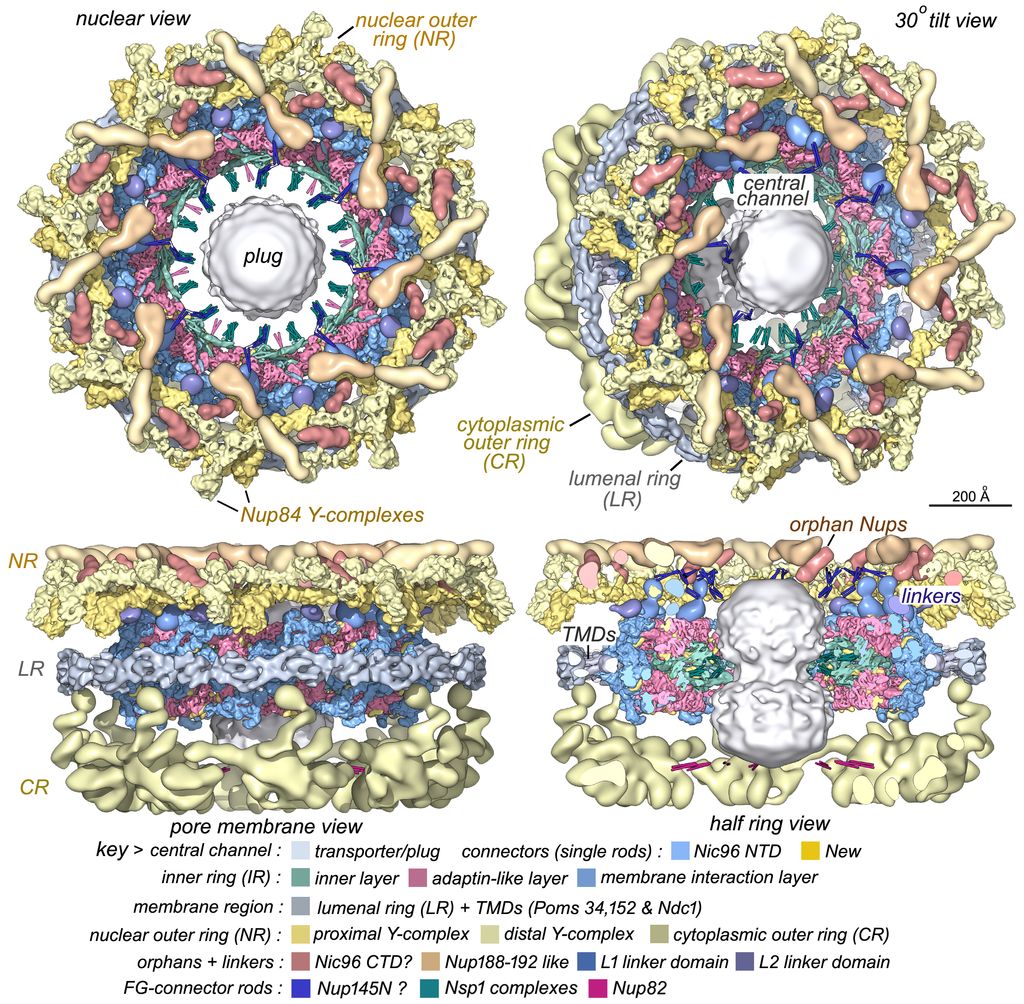
We extended our work on the isolated NPC with cryogenic-EM and single particle methods to provide a composite, multiscale structure of the 8-fold symmetric core scaffold. To date, we have analyzed yeast NPCs from growing cells engaged in transport, which contain a plug-like feature in the central channel comprised of cylindrically-arrayed FG repeats, transport factors and cargo. However, the dynamic core scaffold contracts to a ground state configuration during purification, due to the loss of lateral membrane tension during solubilization of the NE. There are many take home messages from our ongoing work: we now have a better understanding of how these large machines may assemble, how they flex and adapt to changes in transport by expansion of their central passageway, and how Nups have evolved to form 3 different co-axial rings, while some Nups share similar interaction motifs with transport factors that help guide cargo through the central channel. Moreover, multiple NPC isoforms are present in the same cell, which reflects the lego-like ability of this assembly to use interchangeable parts to modify the outer rings. This mutability may play a role in adapting these machines for specialized functions at the nuclear periphery.
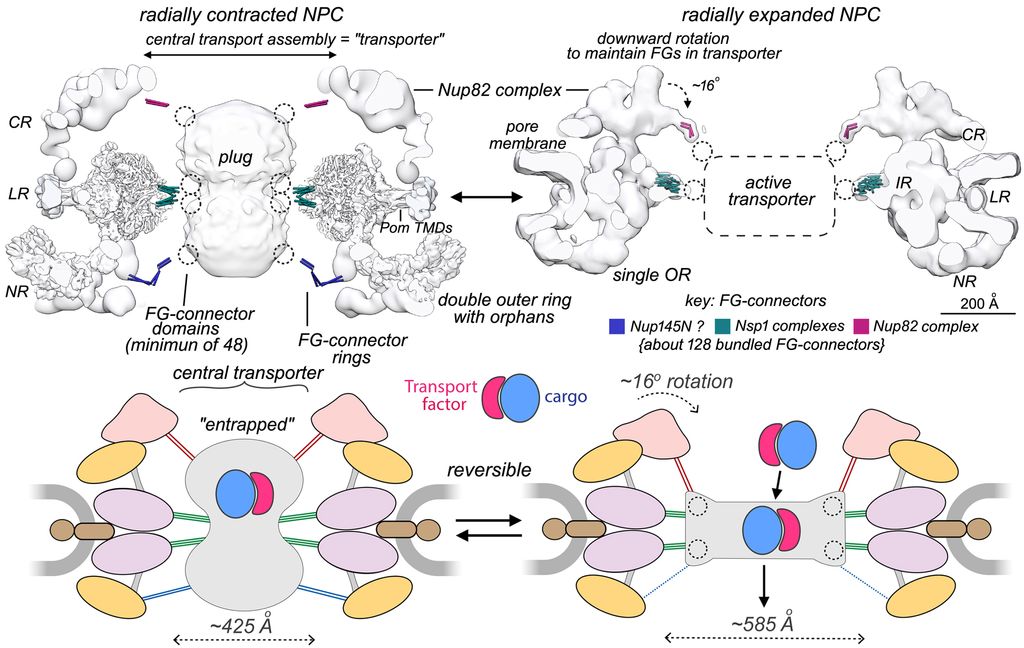
FG-repeats in the central transport channel of the NPC have two functions: they may act as a plug-like barrier that entraps transport factors and cargo complexes in radially contracted NPCs due to their higher packing density, while reorganization of the FG matrix during radial expansion “unplugs” the central channel to facilitate a higher rate of transport. However, the link between the conformation of the core scaffold and the distribution of FG-repeats in the central channel may be modulated by transport factor-cargo complexes and lateral tension in the NE.
Summary: A detailed understanding of the architecture and mechanics of the NPC is providing an in-depth view of how the nucleus and cell body communicate, which underscores an important range of biology and disease processes.
This project is a team effort and major collaborators include Michael Rout, PhD and Brian Chait, PhD (Rockefeller University), Javier Fernandez-Martinez, PhD (Basque Foundation of Science); Ignacia Echeverria, PhD and Andrej Sali, PhD (University of California, San Francisco) Steve Ludtke, PhD (Baylor College of Medicine) and Elizabeth Villa, PhD, (University of California, San Diego).
Molecular Cell Editors choice: 20 papers from 2023> yeast NPC
Previews and paper links –
[Nature] Integrative structure and functional anatomy of a nuclear pore complex.
An Architectural Guide to the Nuclear Pore Complex – NIH Director’s Blog (2018)
New Model of Yeast Nuclear Pore Complex Revealed (2022)
[Molecular Cell] Implications of a multiscale structure of the yeast nuclear pore complex.
Selected NPC Publications:
Akey CW, Echeverria I, Ouch C, Nudelman I, Shi Y, Wang J, Chait BT, Sali A, Fernandez-Martinez J, Rout MP. (2023). Implications of a multiscale structure of the yeast nuclear pore complex. Molecular Cell 83:3283-3302.
Akey CW, Singh D, Ouch C, Echeverria I, Nudelman I, Varberg JM, Yu Z, Fang F, Shi Y, Wang J, Salzberg D, Song K, Xu C, Gumbart JC, Suslov S, Unruh J, Jaspersen SL, Chait BT, Sali A, Fernandez-Martinez J, Ludtke SJ, Villa E, Rout MP. (2022). Comprehensive structure and functional adaptations of the yeast nuclear pore complex.
Cell 185:361-378. doi: 10.1016/j.cell.2021.12.015. PMID: 34982960.
Kim SJ, Fernandez-Martinez J, Nudelman I, Shi Y, Zhang W, Raveh B, Herricks T, Slaughter BD, Hogan JA, Upla P, Chemmama IE, Pellarin R, Echeverria I, Shivaraju M, Chaudhury AS, Wang J, Williams R, Unruh JR, Greenberg CH, Jacobs EY, Yu Z, de la Cruz MJ, Mironska R, Stokes DL, Aitchison JD, Jarrold MF, Gerton JL, Ludtke SJ†, Akey CW†, Chait BT†, Sali A†, and Rout MP†. (2018). Integrative structure and functional anatomy of a nuclear pore complex.
Nature. 555:475-482. doi: 10.1038/nature26003. Epub 2018 Mar 14. PMID: 29539637.
†Co-last authors
Yang, Q., Rout, MP and Akey CW (1998). Three-dimensional architecture of the isolated yeast nuclear pore complex: Functional and evolutionary implications. Molecular Cell 1, 223-234. PMID: 9659919.
Akey CW and Radermacher M (1993). Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. The Journal of Cell Biology 122, 1-19. 10.1083/jcb.122.1.1.
NPC PubMed publications list
NPC EMDB entries
Early Projects
Additional Research Projects in the Lab
- Classic NPCs
- The apoptosome: a cell death signaling platform
- Mammalian and bacterial Ribosome-channel complexes
- The Type IVb secretion system of Legionella pneumophila
- The nucleoplasmin histone chaperone family
Contact Us
Department of Pharmacology, Physiology & Biophysics
Boston University Chobanian & Avedisian School of Medicine
700 Albany Street, W315A
Boston MA 02118-2526
Phone:(617) 358-8451
e-mail: cakey@bu.edu