The Type IVb secretion system of Legionella pneumophila
The T4bSS translocates effector proteins into the host eukaryotic cell. This process is required for infectivity by Legionella, a facultative pathogen. When alveolar macrophages are infected, this leads to a severe pneumonia (Legionnaires’ disease), which is particularly deadly in hospital burn units. We are studying the T4bSS with the goal of creating a molecular model of its function. As a first step, we determined the crystal structure of the interacting domains of IcmR-IcmQ (Rm-IcmQ). IcmR is a chaperone for IcmQ which is able to bind to the membrane surface, while the C-terminal Qc domain binds NAD+, presumably through a scorpion motif. This may be part of a signaling process that occurs at the bacterial plasma membrane to sense the metabolic state of Lp cells as they approach stationary phase, when the T4bSS is fully assembled. We are now focusing on other components of this protein translocase.
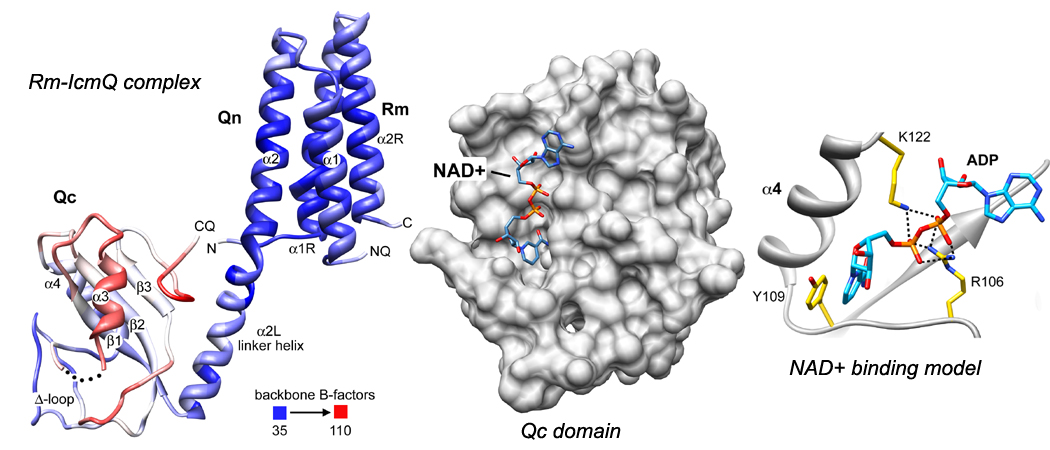
*Structure of IcmR(Rm)-IcmQ and NAD+ binding to the Qc domain
Selected Publications:
Farelli, J.D., Gumbart, J.C., Akey, I.V., Hempstead, A., Amyot, W., McKnight, C.J., Head, J.F., Isberg, R.R. and Akey, C.W. (2013). IcmQ in the Type 4b secretion system contains an NAD+ binding domain. Structure 21,1361-1373. PMID: 23850453
Raychaudhury, S., Farelli, J., Montminy T.P., Ménétret, J.F., Matthews, M., Roy, C.R., Head, J.F., Isberg, R.R. and Akey, C.W. (2009). The structure and function of interacting domains of IcmR-IcmQ from the Type IVb secretion system of Legionella pneumophila. Structure 17, 590-601. PMID: 19368892