Laboratory of Molecular Neurobiology
Welcome to the Laboratory of Molecular Neurobiology at the Boston University School of Medicine. The members of the laboratory are working together as a team under the direction of David H. Farb, PhD, to elucidate the mechanisms by which receptors are regulated, how activation of these receptors modulates neural network activity in vivo and, how this in turn contributes to the onset and progression of memory deficits in age-related neurodegenerative diseases. The laboratory uses a systems neuroscience approach to understanding the functional interactions within neural networks implicated in neurological and psychiatric diseases. Drs. David Farb and Marcia Ratner of the Laboratory of Molecular Neurobiology were one of the first teams of investigators to successfully use in vivo electrophysiological techniques to advance our understanding of how systemically administered drugs modulate neural network activities implicated in memory deficits associated with age-related amnestic mild cognitive impairment (Hippocampus, 2015) and Alzheimer’s disease (AD) (Heliyon, 2021). The Laboratory was the first to identify baseline ripple band hyperactivity as measured by increased average peak ripple amplitude (a core component of local field potential power in the ripple band) during periods of quiet wakefulness in the TgF344-AD rat model of AD (see panels F and G from our 2021 Heliyon paper). The Laboratory was also the first to demonstrate that activity in this high frequency band which, is implicated in memory function in animals and humans, does not increase further when the neural circuitry is challenged with an investigational therapeutic (i.e., α5IA) designed to improve memory by decreasing tonic inhibition mediated by α5 subunit containing GABA-A receptors which are highly expressed in the hippocampus. Drs. Farb and Ratner are also collaborating to advance the use of in vivo electrophysiology as an applied science in preclinical neurotoxicology research (Frontiers in Toxicology, 2022). |
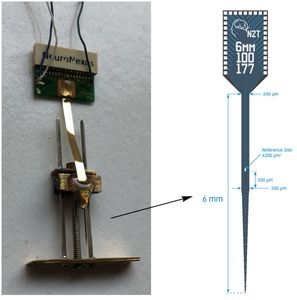
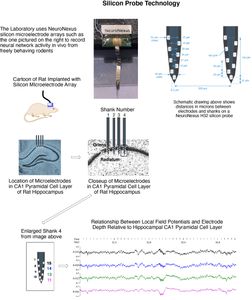
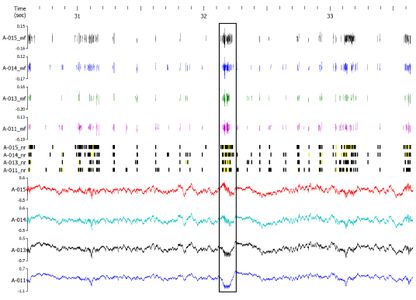
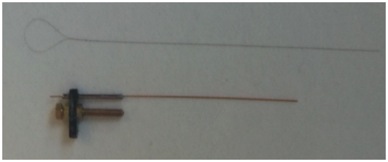
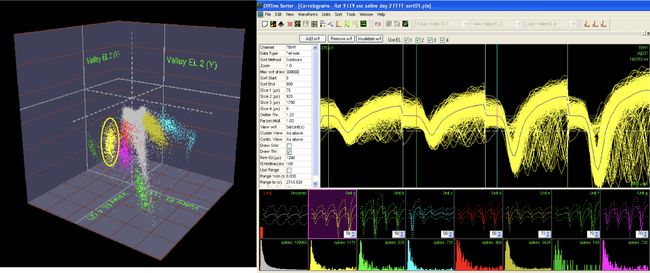
Silicon Probe MicroelectrodesThe Laboratory is currently using ultra fine silicon microelectrode arrays (see figures and video below) to identify subregion specific changes in local field potentials and single unit activity that underlie cognitive deficits associated with aging and disease and, to assess for the functional neural network correlates associated with effective therapeutics for Alzheimer’s disease. Due to their unique design and flexible electrode layouts, silicon microelectrode arrays permit for recording across strata within a hippocampal subregion and, from more than one subregion (e.g. dentate gyrus, CA3 and CA1). Long single shank silicon probes such as the one pictured below permit recording from multiple hippocampal subregions with different current sources and sinks. The figures below demonstrate how local field potentials are recorded and visualized for analysis. Single unit activity associated with high frequency (140 to 200 Hz) sharp wave ripples in the CA1 hippocampal subregion are shown inside box in lower figure. Real-time recordings of ripples with associate action potential “spikes” are shown in the video below titled “Recording of Ripples and Action Potential Spikes in Real-Time“. The data shown in this video were acquired using a 4 shank 32 electrode silicon microlectrode array while the animal was awake and immobile in a familiar environment as reported in our paper published in Cell Press Heliyon, 2021. This research is funded in part by the National Institute on Aging. The Laboratory of Molecular Neurobiology is part of the Boston University Center for Systems Neuroscience. Sharp Wave Ripple with Associated Multiple Unit Activity |
Silicon Probe Microelectrode Arrays
Recording of Ripples and Action Potential Spikes in Real-Time
Place CellsIn vivo electrophysiological recordings have been used to study neuronal activity and hippocampal dependent learning and memory function since the Nobel Prize winning discovery of “Place Cells” by O’Keefe and Dostrovsky in 1971. Place cells, are hippocampal pyramidal cells that fire with a greater frequency when an animal is in a specific location. This activity pattern can be visualized in the video below of the Dancing Place Cells which was made from actual data recordings of hippocampal place cells. |
The Dancing Place Cells Video
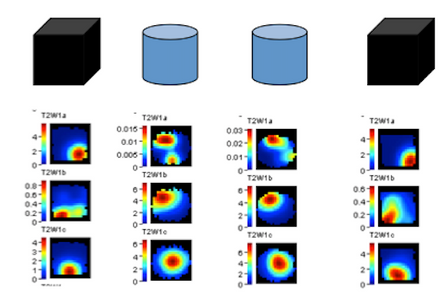
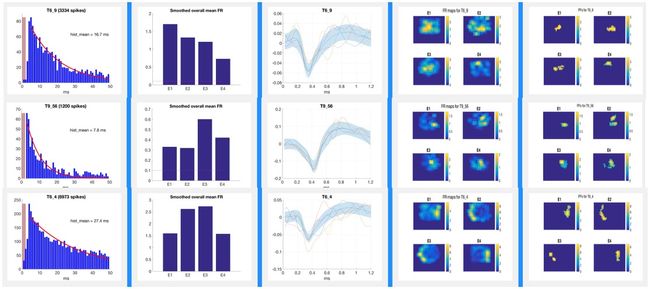
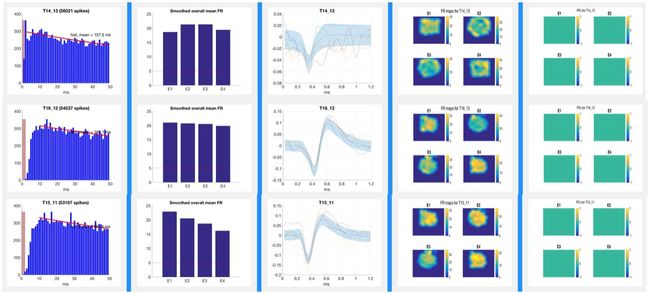
Place FieldsPlace cells establish stable “place fields” within a given environment and change their firing patterns (“remap”) when the animal is moved to a novel location in a classic remapping paradigm such as that shown in the remapping video below. The place fields of a rat “remapping” when the animal is moved from a familiar square environment to a novel cylindrical one is shown in the figure below. The activity of these place cells is altered by aging and diseases. Place cell activity can also be modulated with drugs (e.g., cognitive enhancers). In recent years, advances in vivo electrophysiology coupled with increased understanding of place cell firing dynamics has opened the door to the use of this technology as a research tool in the preclinical drug discovery process. Pyramidal cells with defined place fields in at least one environment are subsequently analyzed for drug-induced effects on firing rates, spatial correlations across environments, and spatial information content per spike. Place Fields |
Remapping Paradigm
Tetrode Array Construction
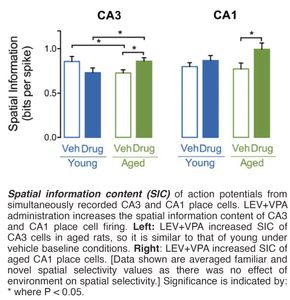
Observations and ResultsCombined Administration of Levetiracetam and Valproic Acid Increases Spatial Information Content Per Spike in Aged Rat Hippocampal Place CellsThe Laboratory has effectively used the methods described above to demonstrate that co-administration of sub-therapeutic doses of the FDA approved anti-epileptic agents levetiracetam and valproic acid reduces hippocampal hyperactivity and increases spatial information content (SIC) per action potential (spike) in the CA3 and CA1 hippocampal subregions of aged rats (see histogram of these data below) (Hippocampus, 2015). |
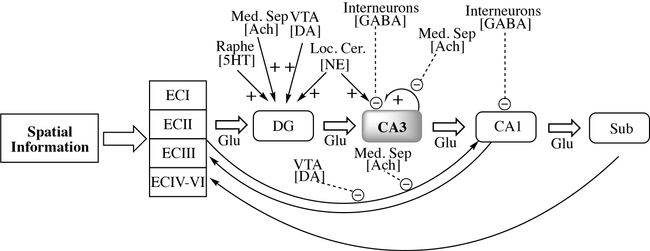
Pharmacological ConnectomePharmacological modulation of hippocampal circuitry implicated in learning and memory: Connectome shows interactions between inhibitory and excitatory inputs. Putative targets for treating age related learning and memory impairments include enhancement of GABA inhibitory tone and attenuation of glutamate mediated excitation. A loss of inhibitory interneurons in the hilar region has been implicated in CA3 pyramidal cell hyperactivity. Acute administration of LEV + VPA may attenuate the hyperactivity in this region of the hippocampus in part by enhancing GABAergic neurotransmission. Muscarinic and nicotinic acetylcholine receptors provide for suppression of feedback excitation and enhancement of afferent input respectively. Ach = acetylcholine; DA = dopamine; DG = dentate gyrus; ECI = entorhinal cortex layer I; ECII = entorhinal cortex layer II; ECIII = entorhinal cortex layer III; GABA = Gamma Amino Butyric Acid; Glu = glutamate; Loc. Cer. = Locus Coeruleus; Med. Sep. = Medial Septum; NE = norepinephrine; Raphe = raphe nucleus; Sub = subiculum; VTA = Ventral Tegmental Area (Hippocampus, 2015). |
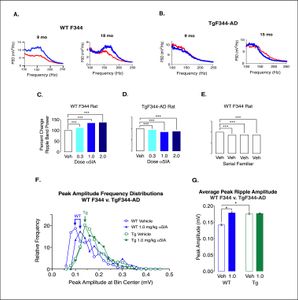
Prodromal dysfunction of α5GABA-A receptors in the TgF344-AD rat model of Alzheimer’s diseaseBioaccumulation of amyloid beta (Aβ) peptides is implicated in Alzheimer’s disease. Animal models over-expressing Aβ show memory deficits. Hippocampal sharp-wave ripples (140 to 200 Hz) have been implicated in memory consolidation. Disruption of these high frequency oscillations produces memory deficits. Compounds that decrease tonic inhibition mediated by α5 type GABA receptors improve memory function in healthy rodents. Administration of the putative cognitive enhancer α5IA which acts as a negative allosteric modulator of α5 type GABAA receptors increases peak ripple amplitudes in wild type adult male rats but has no effect on peak ripple amplitudes in adult male TgF344-AD rats which show elevated plasma concentrations of Aβ42 and Aβ40 (Heliyon, 2021). (see results of these experiments below). |
|
Administration of α5IA does not increase ripple band power or peak ripple amplitude in TgF344-AD Rats. A) Comparison of the spectrums (top panel) from representative well-positioned electrodes in two male F344s age 9 & 18 mo reveals a remarkable overt increase in ripple band power following administration of α5IA (1.0 mg/kg). B) Power spectrums from well-positioned tetrodes adult male TgF344-AD rats age 9–15 months demonstrates a decrease in ripple band power following administration of α5IA. C. Histogram showing percent increase in ripple band power in F344 rats following administration of escalating doses of α5IA. D) Percent decrease in ripple band power in TgF344-AD rats following administration of escalating doses of α5IA. E) Percent decrease in ripple band power in adult F344 rats following administration of vehicle only during serial exposures to a familiar environment. F) Peak ripple amplitude frequency distributions in F344 before and after oral administration of 1.0 mg/kg α5IA (n . 3) show a shift to the right that is not seen in TgF344-AD rats (n . 3). G) Peak ripple amplitude increases in F344 rats following 1.0 mg/kg α5IA. In addition, peak ripple amplitude is significantly greater under vehicle conditions in TgF344-AD rats as compared with wild type F344s. Significance: * at p < 0.05, **p < 0.01, and ***p < 0.001. |
Current Laboratory Members
Select Publications and Abstracts
Laboratory of Molecular Neurobiology in the News
Contact