Sex Differences in Adipose Tissue Biology and Related Metabolic Diseases
INITIATION DATE:
12.01.09
ARC DIRECTORS AND CO-DIRECTORS:
Susan K. Fried, PhD; Director; Professor; Medicine/ Endocrinology
Paul Pilch, PhD; Co-Director; Professor; Biochemistry
OVERVIEW OF GOALS AND MISSION:
Abstract
The ARC focuses on the role of adipose tissue biology in the metabolic complications of obesity in men and women. Our members have complementary expertise in biochemistry, cell biology, immunology and translational research in obesity, diabetes and cardiovascular disease. As a graduating ARC with a close relationship to an existing NIH-funded center, the Boston Nutrition and Obesity Research Center, we have initiated novel collaborations that resulted in interdisciplinary grant proposals, including ‘brite’ adipocytes, adiporedoxin, a novel regulator adipocyte secretory function and adipocyte dysfunction in insulin resistance. We also engaged in fruitful ARC*ARC collaborations with four other ARCS: Mitochondria, Arterial Stiffness, Cancer and Inflammation, and Metabolic Disease and Insulin Resistance: Studies in Patients Undergoing Bariatric Surgery. There is substantial overlap in the membership of these ARCs, which reflect growing collaborations and common interests to enrich the training experiences of graduate and post-doctoral trainees. Thus, we hope to continue to sponsor multidisciplinary seminars and workshops to develop these collaborations and spark new ones.
ARC Members:
| Name/Title (*key personnel) | Dept/School | Role in ARC |
| Susan K Fried* | Medicine, Endocrinology, Diabetes & Nutrition, BUSM | Director |
| Paul Pilch* | Biochemistry, BUSM | Director |
| Caroline Apovian | Medicine, Endocrinology, Diabetes & Nutrition, BUSM | Investigator |
| Tamar Aprahamian* | Medicine, Renal, BUSM | Investigator |
| Barbara Corkey | Medicine, GI, BUSM | Investigator |
| Andrea Coviello | Medicine, Endocrinology, Diabetes & Nutrition, BUSM | Investigator |
| Gerald Denis | Cancer Center, BU | Investigator |
| Stephen Farmer* | Biochemistry, BUSM | Investigator |
| Andrew S. Greenberg* | Tufts U, HNRC | Collaborator |
| Ulla Hansen* | BU-Biology, CRC | Investigator |
| Nawfal Istfan* | Endocrinology, Diabetes & Nutrition BUSM | Investigator |
| Kostya Kandror | Biochemistry, BUSM | Investigator |
| Simon Kasif | Bioinformatics, CRC | Investigator |
| Igor Kramnik | Immunology, BUSM | Investigator |
| Matt Layne | Biochemistry, BUSM | Investigator |
| Mi-Jeong Lee* | Medicine, Endocrinology, Diabetes & Nutrition BUSM | Investigator |
| Karen K. Miller | Endocrinology, MGH | Collaborator |
| Barbara Nikolajczyk | Medicine, Microbiology, BUSM | Investigator |
| Valentina Perissi | Biochemistry, BUSM | Investigator |
| Vishwajeet Puri* | Medicine, Endocrinology, Diabetes & Nutrition BUSM | Investigator |
| Barbara Schreiber | Biochemistry, BUSM | Investigator |
| Steven Smith* | Burnham Inst, Florida Hospital | Collaborator |
| David Waxman | Cell and Molecular Biology, BU-CRC | Investigator |
MAIN ARC PROJECT(S) FOR 2009-2010:
Our group has decided to pursue three objectives that we envision will serve as a new platform to produce preliminary data and evidence of collaborations for planned PPGs.
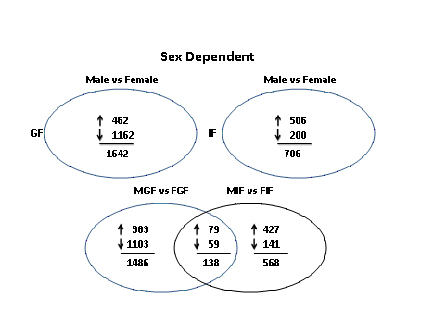
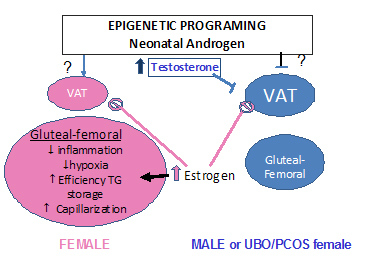
1. Establish and utilize a mouse model (fat transplantation) to determine cell autonomous sex-related differences in adipocyte and adipose tissue biology with a focus on mechanisms underlying the protection of females from adipose tissue inflammation/remodeling events.
2. Identify sex-biased and depot-specific differences in gene expression networks that are common in mouse and human (bioinformatic analysis of existing and emerging datasets).
3. Establish phenotypic characteristics (growth, differentiation) of preadipocytes (adipose stem cells) culture of male and female-derived adipocytes from human adipose depots, and their morphology. Plans for year 2-3 will analyze the metabolic effects of human adipose tissue or preadipocytes of different depot and Mvs. F origin when transplanted into immune-compromised rodents.
ARC AS A RESOURCE:
-Microarray results from visceral adipose tissues of male and female mice and humans
-Adipose Tissues, preadipocyte or other stromal-derived cultures, and their products (e.g conditioned media) from male and female mice and humans
AIM: We plan seminars and activities, co-sponsored by BNORC, to foster the translation of basic research in adipose tissue biology and obesity using cell and mouse models. We focus on topics that bring together multiple ARCs.
– The Annual Program of BNORC will focus on Nutrition, obesity and the microbiome, a topic of keen interest to the Bariatric ARC as well as ours.
– We will also continue seminars around the interface of CVD, Insulin resistance and Obesity in men and women that was highlighted in our recent symposium on Brain Control of Sympathetic Function and Cardiovascular Function.
– Browning, britening, and whitening of adipocytes. This topic involves active collaborations with the MitoARC.
Our central goal is to bring together researchers on the BUMC and BU to accelerate conversations on topics related to obesity and related disease. The goal is innovative integrative collaborations to address this very difficult to study disease at basic and translational levels.
Pictures, Images, Figures: