Molecular, Biomechanical and Genetic Mechanisms of Arterial Stiffness
INITIATED
12.01.10
DIRECTORS
Founding Dir. Richard A. Cohen
Dept Medicine/Whitaker Cardiovascular Institute
Professor and Director, Vascular Biology Section
Co-Dir. Kathleen Morgan, PhD
Dept Health Sciences, College of Health & Rehabilitation Sciences: Sargent College, Charles River Campus
Professor and Chair
kmorgan@bu.edu
617-353-7464
Co-Dir. Francesca Seta, PhD
Dept Medicine, Vascular Biology Section
Assistant Professor
setaf@bu.edu
617-638-7119
Discovery highlight, March 2017:
We have now initiated efforts to identify therapeutic target molecules to prevent or reverse aortic stiffness. We have discovered that activation of the lysine deacetylate sirtuin-1 has potent anti-inflammatory and anti-oxidant effects on the vascular wall decreasing arterial stiffness in a model of diet-induced obesity 1. In addition, we successfully applied GWAS data to identify candidate molecules to develop cell permeant, microbubble targeted decoy peptides that are effective in decreasing aortic stiffness in an aged rodent model 2. Therefore, if translated to humans, sirtuin-1 activators, such as resveratrol or other drugs currently in development, and our targeted decoy peptides represent an attractive therapy against arterial stiffness.
References:
1. Fry, J. L., Al Sayah, L., Weisbrod, R. M., Van Roy, I., Weng, X., Cohen, R. A., Bachschmid, M. M. & Seta, F. Vascular Smooth Muscle Sirtuin-1 Protects Against Diet-Induced Aortic StiffnessNovelty and Significance. Hypertension 68, 775–784 (2016).
2. Nicholson CJ, Seta F, Lee S, Morgan KG. MicroRNA-203 mimics age-related aortic smooth muscle dysfunction of cytoskeletal pathways. Journal of Cellular and Molecular Medicine. 2016, Aug 9. doi: 10.1111/jcmm.12940. [Epub] PMID 27037223.
OVERVIEW OF GOALS AND MISSION, 2010:
Abstract
The Arterial Stiffness ARC has brought together translational research on arterial stiffness and cardiovascular complications. The expertise of API, representing departments at BUMC, CRC, and the Framingham Heart Study spans biomechanical, cell and molecular biological, animal and human pathophysiological models, and epidemiological and genetic mechanisms of stiffness. The ARC has completed nearly 4 years of funding. Major accomplishments from the ARC have led to 1) 28 publications by ARC member/collaborators in the last year, 2) 2 NIH and 1 NSF grant funded in the last year directly related to the arterial stiffness ARC program, and 3) has successfully established major technical capabilities for cardiovascular investigations at BU by establishing core capabilities. The latter, managed by Dr. Seta as ARC fellow includes, 1) an ultrasound and blood pressure core providing stiffness and blood pressure measurements (both non-invasively and invasively) in API’s mouse models, 2) a core to measure biomechanical properties of rodent aorta in vitro, 3) a paradigm to probe human GWAS data to search for genes of interest to ARC API that are associated with stiffness, including BCL11B, adenosine receptors (ADORA), adiponectin, and iPLA2 as promising candidates, and 4) continuing meeting and seminar series that serve as a forum for discussion. Notable amongst the publications in the past year by ARC collaborators are 1) a major study showing that sirtuin-1 prevents aortic dissection and regulates aortic stiffness in response to angiotensin II, 2) a study establishing a novel role for smooth muscle cell cortical actin that actively regulates vascular stiffness, and 3) two population studies establishing the epidemiological link between aortic stiffness and microvascular damage in the brain and kidney.
The success of the ARC is demonstrated in part by over $1.7M in new grants related to arterial stiffness obtained ARC API’s in 2015.
ARC MEMBERS
| Name/Title | Dept/School | |||
| Richard A. Cohen,PhD; Professor | Medicine; CVI | Founding Director | ||
| Kathleen Morgan, PhD; Professor | Sargent College, Charles River Campus | Co-Director | kmorgan@bu.edu | http://sites.bu.edu/morgan-lab/people/lab-members/ |
| Francesca Seta, PhD; Assistant Professor | Medicine | Co-Director | setaf@bu.edu | http://profiles.bu.edu/Francesca.Seta |
| ARC Members: | ||||
| Bela Suki, PhD; Professor | Biomedical Engineering, Charles River Campus | bsuki@bu.edu | ||
| Gary Mitchell, PhD; President | Cardiovascular Engineering, Inc, Norwood, MA | GaryFMitchell@mindspring.com | ||
| Victoria Bolotina, PhD; Professor | Medicine, Vascular Biology | bolotina@bu.edu | http://profiles.bu.edu/Victoria.Bolotina | |
| Barbara Smith, PhD; Professor emeritus | Biochemistry | bdsmith@bu.edu | ||
| Barbara Schreiber, PhD; Associate Professor | Biochemistry | schreibe@bu.edu | http://profiles.bu.edu/Barbara.Schreiber | |
| Matthew Layne, PhD; Associate Professor | Biochemistry | mlayne@bu.edu | http://www.bumc.bu.edu/biochemistry/profiles/matthew-d-layne/ | |
| Xiao Yong Tong, PhD; Assistant Professor | Medicine | |||
| Katya Ravid, DSc/PhD; Professor | Medicine | kravid@bu.edu | http://profiles.bu.edu/Katya.Ravid | |
| Victoria Herrera, MD; Professor | Medicine | vherrera@bu.edu | http://profiles.bu.edu/Victoria.Herrera | |
| Joseph Vita, PhD; Professor | Medicine | |||
| Nelson Ruiz-Opazo, PhD; Professor | Medicine | nruizo@bu.edu | http://profiles.bu.edu/Nelson.RuizOpazo | |
| Katherine Yanhang Zhang, PhD; Associate Professor | Engineering, Charles River Campus | yanhang@bu.edu | ||
| Matt Nugent, PhD; Adjunct Professor | Biochemistry | mnugent@bu.edu | http://profiles.bu.edu/Matthew.Nugent |
ARC AS A RESOURCE
Background:
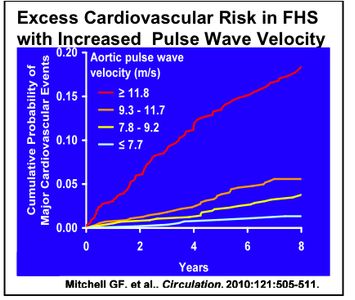
With advancing age or prematurely in obese subjects, arterial stiffness increases and has been implicated in the altered vascular hemodynamics that ultimately lead to the development of hypertension (Mitchell GF, Hypertension 2004). Stiffness begins in the aorta and large arteries, but because artery stiffness transmits higher pressures to the smaller resistance arteries, large artery stiffness is implicated in the microvascular complications of hypertension in the kidney and brain. Data acquired at the Framingham Heart Study (FHS) demonstrate that vascular stiffness is an independent risk factor for cardiovascular disease endpoints (Figure 1), however the mechanisms involved and possible therapeutic approaches remain unclear. Arterial stiffness is assessed in patients by measurements of pulse wave velocity (PWV), and it has been proposed that routine measurements of PWV might provide an earlier and therefore better predictor of cardiovascular disease and preventive treatments. Mechanisms of increased stiffness likely include altered properties of elastin and collagen, as well as increased tone of smooth muscle cells resulting from increased stiffness of the connections between cells and matrix or from endothelial dysfunction. Thus, further investigations into the mechanisms of arterial stiffness will to address the vascular biology of the arterial wall and its component cells – smooth muscle, endothelium, fibroblasts, and bone marrow derived cells.
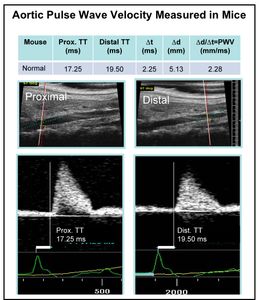
The Arterial Stiffness ARC consists of a group of investigators across both campuses interested in the structural, biochemical, physiological, molecular and genetic mechanisms that underlie arterial stiffness as it relates to negative cardiovascular disease outcomes. With the help of the Animal Ultrasound Core, directed by Dr. Victoria Herrera, the group has established the ability to assess PWV in rodent models of hypertension and arterial stiffness (Figure 2). At the time of initiation, at least 5 such models being studied by ARC members, including obese and aging mice and salt-sensitive rats have been demonstrated to have increased arterial stiffness. For example, in normal mice we had obtained values of approximately 2 mm/msec, which is in the same range as the normal value determined in children, and statistically significant 2- to 2.5-fold increases had been measured in high fat diet-fed and very aged mice, as well as in one novel transgenic mouse.
Another effort initiated by the ARC with Dr. Mitchell was designed to evaluate correlates of genes of interest to the ARC members with SNP’s in those genes determined to correlate with increased arterial stiffness in a multicenter, genome wide association study.
Rationale and AIMS of research as the ARC began:
1. To measure arterial stiffness assessed by alterations in pulse wave velocity in rodent models hypothesized by API’s to have arterial stiffness based on their previous studies on the protein or disease. An expanding number of ARC investigators will be able to have PWV measured in their various mouse models that they believe might have arterial stiffness, thereby achieving pathological significance for their gene or protein of interest.
2. To measure gene expression in mouse aortas that prove to be stiff in Aim #1 using RTPCR array chip technology in the BU chip core. The genes that are most important in mediating in the integrated development of stiffness are unknown. We hope to detect novel genes important for stiffness in two mouse models of stiffness, obese and aged mice, and in that way stimulate further study by the ARC members.
3. To collaborate with Gary Mitchell to test if genes of interest to ARC members may contain polymorphisms detected in a multicenter genome wide association study of arterial stiffness in ~30,000 human subjects. Detecting SNP’s in genes of interest to ARC members is seen as important for further development of their research programs and for establishing significance of their genes of interest to arterial stiffness in humans.
4. Make biomechanical measurements of stiffness to directly assess in vitro aortic stiffness in mice shown to have increased arterial pulse wave velocity. These measurements are seen as necessary to link in vivo properties of stiffness to biomechanical, and therefore biochemical and molecular mechanisms. An obvious reason is to discriminate whether stiffness in various models results from active (i.e. smooth muscle tone) or passive structural components. These experiments will also allow determination of endothelial contributions and coordination with biochemical measurements.
5. Seminar speakers on topics related to clinical and experimental arterial stiffness, hypertension, matrix, smooth muscle, and endothelium will be invited to increase knowledge and awareness of the group, and a mini-symposium will be held on the topic.
Research updates, 2014 – 2015:
The Arterial Stiffness ARC was conceived as a cooperative work group to enable collaborative studies on this exciting area of interest within the BU community. The ARC existed as a preARC during 2009-2010. During this period Gary Mitchell with BU collaborators (Hamburg, Vita, Benjamin, Vasan, Levy) has published two key papers based on Framingham Heart Study results demonstrating that arterial stiffness in the general population is an independent predictor of cardiovascular morbidity (Figure 1) 1, and predicts the later onset of arterial hypertension2. Mitchell with Vasan Ramachandran, another ARC member received a new RO1 in 2015 to further study the implications of these findings. These important results provide the rationale for studies by ARC members of the poorly understood biochemical, biophysical, biomechanical, and genetic mechanisms of vascular stiffness. Also, encouraged by the pre-ARC and coincident with ARC initiation, two members of the pre ARC (Cohen and Ruiz-Opazo) each had RO1’s funded by the NIH initiative on rodent models of arterial stiffness, giving a tremendous boost to the local level of activities related to the focus of the ARC. This gave important access to the knowledge base and activities of the 10 NIH-centers similarly funded to study arterial stiffness. Six of the outside PI’s funded at these centers (Berkowitz, Leopold, Mecham, Wagenseil, Harrison, Larson) have presented their work at ARC seminars during the 3 years of this ARC. ARC activities have been in 4 areas, 1) development of biomechanical methods for direct measurements of arterial stiffness of isolated aortas of ARC members genetic mouse models, 2) explorations of genes of interest to ARC members within the human arterial stiffness GWAS database established by Gary Mitchell, and development of a 96-gene qRTPCR mouse gene chip to test members’ mouse tissue samples, 3) establishing the expertise and resources to measure arterial stiffness measurements of aortic pulse wave velocity by ultrasound and high-fidelity pressure catheters in members’ mouse models, and 4) monthly seminars and ARC meetings. During the last year the ARC has been an ARC program and funds have gone to 1) seminars, 2) a major effort of building and participating in core activities to measure arterial stiffness and blood pressure in rodents to stimulate grant submissions, and 3) to further establish preliminary work on the role of sirtuin-1 in regulating aortic stiffness and dissection.
Major scientific progress was made in discovery of the active role of smooth muscle in aortic stiffness by the Morgan lab (Figure 2). The
novel proposal that tyrosine phosphorylation events in the actin cytoskeleton regulate stiffness at the macro level led to funding of an R21 to Morgan and co-investigator Seta in 2015.
Another major scientific outcome of the ARC program were cooperative studies of Seta, Morgan, Colucci, and Cohen on the role of sirtuin-1 in aortic stiffness. A recent paper (Fry et al. 2015) established that sirtuin-1 normally prevents aortic stiffening and dissection during angiotensin II-induced hypertension. These findings led to studies amongst the co-authors on acetylation of cytoskeletal proteins and the role of sirtuin-1 in aortic stiffness due to obesity. These studies showed that transgenic mice with cell-specific increased smooth muscle sirtuin-1 expression are prevented from high fat diet-induced stiffness. In addition, a sirtuin-1 activator, SRT1720, had the surprising ability to reverse aortic stiffness to the normal range within 2 weeks of administration (Figure 3). The ARC investigators are working on grant submissions related to these findings now.
Drs. Seta and Gary Mitchell are working on another important outcome of ARC supported work, the finding reported last year that mice with genetic deficiency in BCL11b have aortic stiffness. BCL11b is the nearest gene to the single nucleotide polymorphism with genome wide association to aortic stiffness. New findings confirmed that BCL11b deficient mice have increased aortic stiffness, and that surprisingly, the aorta highly expresses this gene and protein whose only known role is as a T-cell antigen.
Research updates, 2009 – 2013:
1. Biomechanical measurements: Kathy Morgan and Bela Suki collaborated to establish methods and adapt them to the challenge of making direct stiffness measurements in mouse aorta in vitro. A PhD student (Tony Gao, Bioengineering) was funded by the ARC since May 2011. Suitable instrumentation devoted to ARC activity was purchased and is being used by Tony Gao under the supervision of Drs. Morgan and Suki. Mouse aortas are physically mounted and stiffness is assessed from the slope of the stress-strain relationship during oscillations in length, as shown in Figure 2. A second protocol has also been developed to assess arterial stiffness in response to small amplitude stretch oscillations, which mimic the strain the blood vessels are subjected to during the cardiac cycle and therefore more reflective of in vivo, physiological conditions. New data collected by Tony Gao with both methods show a greater inhibition of stiffness by PP2 in young mouse aorta, pointing to an age-dependent role of src-dependent tyrosine kinase signaling in young vs old mouse aortic smooth muscle. This observation illustrates a novel area of ARC supported research on the role of smooth muscle tone in arterial stiffness.
2. Stiffness genes: Gary Mitchell remains an important resource to ARC PI’s because of his experience in the genetics of arterial stiffness. Because of his contributions, he received a “Collaborator of the Year” award from the Evans Center in October 2012.
In his recently published study3, genome wide significance for an association with stiffness was achieved for single nucleotide polymorphisms (SNP’s) at only one gene locus, near the T-cell antigen gene, BCL11B. This finding related to stiffness in the human population led the ARC to include BCL11B in its qRTPCR gene chip analysis of API mouse models, and is responsible for preliminary observations in mouse models of two API’s of altered aortic BCL11B expression. In addition, the ARC obtained floxed BCL11B and BCL11B T-cell deficient mice from Dr. Dorina Avram at Albany Medical Center, These mice have entered a protocol to determine if aortic stiffness is altered before or during high fat diet feeding, an intervention the ARC has used in other API’s mice (see below). Preliminary results indicate that BCL1B11B deficient mice have increased PWV compared to controls. Aside from the obvious interest in whether these mice have altered arterial stiffness, when the mice are sacked, tissues will be analyzed for genes of interest of API by qRTPCR, and biomechanical properties will be measured in the aorta. Depending on compelling results, API mice with altered stiffness could be bred with the BCL11B deficient mice. Floxed BCL11B mice are currently being re-derived by LASC and will be available to BU investigators to generate cell-specific or conditional BCL11B mutant mice.
In parallel, an alternative analytical approach was investigated whereby ARC members submitted genes to Dr. Mitchell in sets of 5 – 50 genes that were screened for significant change within the PWV SNP database, thereby reducing the burden of second order statistical error in the genome wide analysis. Thus far, four screens were submitted and resulted in 3 genes that achieved statistical significance within their respective subsets for association with stiffness in the human database. These include ADORA1, an adenosine receptor, submitted by Dr. Ravid. As a result of this finding, the ARC determined stiffness in various mice provided by Dr. Ravid with genetic alteration in adenosine receptors and obtained exciting data (see below). Another hit in the human GWAS search was the mitochondrial regulatory protein, mitofusin-2 (MFN-2). As the mito ARC has been developing a tissue-specific MFN2 transgenic mouse which is nearing readiness, Dr. Seta plans to breed smooth muscle specific MFN2 knockout mice to determine the effect on stiffness.
The ARC used a qRTPCR gene array in which 96 mouse genes of interest to API were screened against mRNA obtained from tissue of ARC members’ mouse models. Results of this array included preliminary evidence of altered BCL11B and Src expression in the aorta of mice of two API. Much additional data were obtained, including that showing inflammatory and fibrotic gene expression were elevated in stiffer aortas. The example shown in figure 3 indicates a 2-fold increase in the expression of TGFβ in aorta of mice fed high fat diet for 5 months that is reversed, along with the stiffness induced, by reversion to normal diet for 2 months.
3. Stiffness and Blood Pressure Measurements in Mice: The ARC has established a program whereby members can have aortic PWV measured by VEVO770 ultrasound in their live mice that they suspect may develop arterial stiffness. Based on published methodology4, during the last year, the ARC worked to significantly enhance the time consuming analysis, by working with Dr. Mitchell to obtain custom hardware and software for the analysis. The ARC has set up and optimized methods for the use of this custom made acquisition and analysis workstation that can interface with the VEVO770 to directly acquire and/or analyze previously acquired blood flow waveforms to automatically compute PWV. In addition, the ARC has acquired three high-fidelity pressure catheters for invasive PWV and concomitant blood pressure measurements in anesthetized mice. Radiotelemetry equipment is also in place to measure blood pressure in conscious mice. All together, these technologies allow complete, accurate and state-of-the-art assessment of cardiovascular phenotype of mice in vivo. This “cardiovascular core” will remain available to BU investigators on a collaborative basis with the goal of acquiring preliminary data for grants and publications. This year the ARC performed measurements on ADORA2b -/- (K. Ravid), and additional 21 mo old (K. Morgan), adiponectin -/- (T. Aprahamian and K. Walsh), Nox4 endothelial dominant negative transgenic (X. Tong), iPLA2 mutant (V. Bolotina) and female/male mice (S. Fried). Major findings this year included finding that at 3 months of age, ADORA 2b -/- mice have increased aortic stiffness equivalent to that of 21 month old mice (Figure 4). We also found that the increase in aortic stiffness after 8 months of high fat diet (HFHS), identified last year, was prevented in adiponectin transgenic mice and possibly augmented in the knockout (Figure 4). These studies have identified two novel mouse models of arterial stiffness.
4. Seminars and ARC meetings: During the three years of activites the ARC has had numerous seminars given by outside speakers and renowned experts in arterial stiffness (Humphrey, Seals, DeCabo, Wegenseil, Larson, Harrison, Fleenor, Lakatta) and by ARC members (Zhang, Mitchell). Two of the seminars by outside speakers took place on the CRC (Humphrey, Reinhart-King) and three were co-sponsored with the Whitaker Cardiovascular Institute (Harrison, Fleenor and Lakatta). In addition, ARC meetings have focused on major ARC initiatives during which planning, results, and feedback on ARC projects have been discussed.
Abstracts presented in meetings:
Arterial stiffness in diet-induced obese mice is reversed by weight loss. Tina Shiang, Leona Al Sayah, Jessica Fry, Robert M. Weisbrod, Saumendra Bajpai, Cynthia Reinhart-King, Richard A. Cohen and Francesca Seta. (Experimental Biology Annual Meeting 2013).
Arterial stiffness in diet-induced obese mice is reversed by weight loss. Leona Al Sayah, Liana Rubinoff, Tina Shiang, Robert M. Weisbrod, Saumendra Bajpai, Cynthia Reinhart-King, Richard A. Cohen and Francesca Seta (Evans Research Day 2013 Poster; third place winner).
Changes in cellular and extracellular mechanisms of arterial stiffness with aging. Gao YZ, Saphirstein RJ, Suki B, Morgan KG. “” (Biomedical Engineering Society Conference, 2013).
Changes in cellular and extracellular mechanisms of arterial stiffness with aging. Gao YZ, Saphirstein RJ, Suki B, Morgan KG. (Evans Reseach Day 2013).
The focal adhesion: a regulated component of aortic stiffness. Saphirstein RJ, Gao YZ, Jensen MH, Gallant CM, Vetterkind S, Moore JR, Morgan KG. “ (Experimental Biology Annual Meeting 2013).
Regulation of venous stiffness by smooth muscle cell-matrix adhesions. Saphirstein RJ, Gao YZ, Moore JR, Morgan KG. (North American Vascular Biology Organization’s Vascular Biology Conference 2013).
Regulation of venous stiffness by smooth muscle cell-matrix adhesions. Saphirstein RJ, Gao YZ, Moore JR, Morgan KG. (Evans Research Day 2013).
Publications:
Arterial Stiffening Precedes Systolic Hypertension in Diet-induced Obesity. Weisbrod M.R., Shiang T., Al Sayah L., Fry L.J., Bajpai S., Reinhart-King A.C., Lob E.H., Santhanam L., Mitchell G., Cohen R.A. and F. Seta. Hypertension 2013; 62: 1105-1110.
The Contribution of Vascular Smooth Muscle to Aortic Stiffness Across Length Scales. Saphirstein R.J. and K.G. Morgan. Microcirculation 2013 (invited review).
Vasoconstrictor-induced Endocytic Recycling Regulates Focal Adhesion Protein Localization and Function in Vascular Smooth Muscle. Poythress RH, Gallant C, Vetterkind S, Morgan KG. Am J Physiol Cell Physiol. 2013 Jul;305(2):C215-27. doi: 10.1152/ajpcell.00103.2013. Epub 2013 May 22. PMID: 23703522
The Focal Adhesion: a Regulated Component of Aortic Stiffness. Saphirstein RJ, Gao YZ, Jensen MH, Gallant CM, Vetterkind S, Moore JR and KG Morgan. PLoS One. 2013 Apr 23;8(4):e62461. doi: 10.1371/journal.pone.0062461. Print 2013. PMID: 23626821.
Relations of Arterial Stiffness and Endothelial Function to Brain Aging in the Community. Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Neurology 2013 Aug 9 (e-pub ahead of print).
Aortic stiffness, blood pressure progression, and incident hypertension. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. JAMA 2012; 308:875-881.
Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O’Donnell CJ, Mitchell GF, Vasan RS. Circulation 2012; 125:2836-2843.
Circulating vascular growth factors and central hemodynamic load in the community. Zachariah JP, Xanthakis V, Larson MG, Vita JA, Sullivan LM, Smith HM, Safa R, Peng X, Hamburg N, Levy D, Sawyer DB, Mitchell GF, Vasan RS. Hypertension 2012; 59:773-779.
Circulating angiogenic cell populations, vascular function, and arterial stiffness. Cheng S, Wang N, Larson MG, Palmisano JN, Mitchell GF, Benjamin EJ, Vasan RS, Levy D, McCabe EL, Vita JA, Wang TJ, Shaw SY, Cohen KS, Hamburg NM. Atherosclerosis 2012; 220:145-150.
Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE, Blumberg JB, Vita JA. Am J Clin Nutr 2011; 93:934-940.
Effects of Concord grape juice on ambulatory blood pressure in pre-hypertension and stage 1 hypertension. Dohadwala MM, Hamburg NM, Holbrook M, Kim BH, Duess M-A, Levit A,Titas M, Chung WB, Vincent FB, Caiano TL, Frame AA, Keaney JF, Vita JA. Am J Clin Nutr 2010; 92:1052-1059.
Arterial stiffness and cardiovascular events: The Framingham Heart Study. Mitchell GF, Hwang S-J, Ramachandran VS, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Circulation 2010; 121:505-511.
Effects of basic calponin on the flexural mechanics and stability of F-actin. Jensen MH, Watt J, Hodgkinson J, Gallant C, Appel S, El-Mezgueldi M, Angelini TE, Morgan KG, Lehman W, Moore JR. Cytoskeleton 2012 69:49-58.
Structure and Dynamics of the Actin-Based Smooth Muscle Contractile and Cytoskeletal Apparatus. Lehman W, Morgan KG. Journal of Muscle Research and Cell Motility, in press 2012.
Src Modulates Contractile Vascular Smooth Muscle Function via Regulation of Focal Adhesions. Min J, Reznichenko M, Poythress P, Gallant C, Vetterkind S, Li Y, Morgan KG. J Cell Physiol. 2012 227: 3585-92. PMID: 22287273 PMC3348426
Non-redundant roles of cytoplasmic beta- and gamma-actin isoforms in regulation of epithelial apical junctions. Baranwal S, Naydenov NG, Harris G, Dugina V, Morgan KG, Chaponnier C, Andrei I. Ivanov AI. MBoC 2012 in press.
A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Koupenova M, Johnston-Cox H, Vezeridis A, Gavras H, Yang D, Zannis V, Ravid K. Circulation, 2012 Jan 17;125(2):354-63. PMCID: PMC3265935.
A2 adenosine receptors and vascular pathologies. Johnston-Cox HA, Koupenova M, Ravid K. Arterioscler Thromb Vasc Biol. 2012 Apr;32(4):870-8. Review. PMID: 22423039.
The A2b adenosine receptor modulates glucose homeostasis and obesity. Johnston-Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG LeBrasseur N, Ravid K. PLoS One. 2012;7(7):e40584. Epub 2012 Jul 25. PMCID: PMC3405065.
Regulation of atherosclerosis and associated risk factors by adenosine and adenosine receptors. Koupenova M, Johnston-Cox H, Ravid K. Curr Atheroscler Rep. 2012 Oct;14(5):460-8. PMID: 22850979.
Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Durik M, Kavousi M, van dP, I, Isaacs A, Cheng C, Verdonk K, Loot AE, Oeseburg H, Bhaggoe UM, Leijten F, van Veghel R, de Vries R, Rudez G, Brandt R, Ridwan YR, van Deel ED, de Boer M, Tempel D, Fleming I, Mitchell GF, Verwoert GC, Tarasov KV, Uitterlinden AG, Hofman A, Duckers HJ, van Duijn CM, Oostra BA, Witteman JC, Duncker DJ, Danser AH, Hoeijmakers JH, Roks AJ. Circulation. 2012;126:468-478.
Electromechanical and structural alterations in the aging rabbit heart and aorta. Cooper LL, Odening KE, Hwang MS, Chaves L, Schofield L, Taylor CA, Gemignani AS, Mitchell GF, Forder JR, Choi BR, Koren G. Am J Physiol Heart Circ Physiol. 2012;302:H1625-H1635.
Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Brain. 2011;134:3398-3407.
Common genetic variation in the 3′-BCL11B gene desert is associated with carotid-femoral pulse wave velocity and excess cardiovascular disease risk: the AortaGen Consortium. Lead author: Mitchell GF, and 30-40 other authors including from BU..,Vasan RS, … Benjamin EJ, …Levy D, … . Circ Cardiovasc Genet. 2012;5:81-90.
Aortic stiffness and cerebral blood flow. Mitchell GF. Am J Hypertens. 2011;24:1056.
Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. ……Mitchell GF, …. Levy D, (amongst >25 authors) Nat Genet. 2011;43:1005-1011.
Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Circulation. 2010;122:1379-1386.
Arterial Stiffness and Wave Reflection: Biomarkers of Cardiovascular Risk. Mitchell GF. Artery Res. 2009;3:56-64.
Goals:
This ARC will use novel approaches to study the emerging concept that cardiovascular disease can be detected early by noninvasive measurements of arterial stiffness. The ARC members constitute members of the BU community with diverse expertise in vascular biology, hypertension, smooth muscle physiology, extracellular matrix, biomechanics, and genetics for which the concept of arterial stiffness represents increased significance for their own areas of focus. We will apply novel methods by 1) establishing mouse models of increased pulse wave velocity in order to make the translational link between human measurements and those in the rodent in which mechanisms can be studied, 2) measuring arterial stiffness directly in isolated rodent aortas order to make the link from in vivo measurements to active and passive properties of the blood vessel in order to establish biochemical and biomechanical mechanisms, 3) detecting novel genes of interest by probing aortas of obese and aging mice, 4) determining if expression of genes in which polymorphisms are identified to be associated with arterial stiffness are also affected in rodents with stiffness. The significance of ARC studies is to establish rodent models of stiffness in which mechanisms and biomarkers of stiffness in humans might be confirmed. Therapeutic approaches can then be tested by ARC members in these rodent models.
1: ARC funds will be used to support small animal ultrasound core measurements and analyses of vascular stiffness in rodents provided by ARC members other than those who already have funded grants to do so (ie. Cohen, Ruiz-Opazo). Dr. Herrera and Cohen will further refine methods for these measurements together with our consultant, Gary Mitchell. Ten API currently have or are developing mouse or rats that they hypothesize might have arterial stiffness that will need to be screened.
2: mRNA will be isolated from aortas of obese and aging mice during the first 3 months of the ARC so that data can be made available for ARC members during the first year.
3: ARC members will develop lists of genes of interest focused on each of the research areas of ARC members and submitted to Dr. Mitchell. While the results of his GWA study are currently unpublished, Dr. Mitchell has indicated many significant changes in genes have been identified. By submitting limited numbers of genes and by grouping them according to function, we hypothesize that additional significant associations of polymorphisms in genes of interest will be acquired by limiting second order statistical error.
4: Dr. Bela Suki, an expert in biomechanical measurements of stiffness in Biomedical Engineering on the CRC has been recruited by the ARC. ARC funds will support a part time student to use equipment already present in his lab to measure stiffness in rodents identified by ARC members to have increased pulse wave velocity. In addition, preliminary studies by Dr Tsui and Dr. Morgan will begin to develop the use of atomic force microscopy and optical tweezers to measure stiffness of single isolated smooth muscle cells and their connections to the matrix.
5: Seminar speaker travel and accommodation expenses will be supported by the ARC.
Collaborative studies will continue to develop improved methods for stiffness measurements as it continues to be measured in live rodents and in vitro on isolated rodent aortas of ARC members. We expect that ARC members will develop collaborations together. For instance, Dr. Morgan and Tsui will develop methods to measure stiffness in individual cells. In their funded programs Drs. Cohen, Ruiz-Opazo, and Herrera use both tail cuff and telemetry blood pressure measurements, and as members identify stiffness in their rodent models, they can collaborate with these investigators to have blood pressure evaluated in their rodents to determine if stiffness is associated with hypertension. Examples of other potential collaborative efforts are 1) use of immunohistochemistry core facilities to determine changes in matrix or protein expression in stiff aortas, 2) development of nanoparticles for local delivery of genes or drugs expected to improve stiffness, 3) with identification of mice with alterations in the expression of genes identified in the human studies, the members can obtain transgenic mice of their interest and have stiffness measurements in them using ARC funds. It is also evident to all ARC members that the topic of vascular stiffness encompasses the interest of a large number of basic, translational, and clinical investigators on both the BUSM and CRC campuses, making it an ideal topic for programmatic development within the BU community.
Reporting:
ARC progress meetings will continue monthly. Members who have stiffness studies performed using ARC funds will present their findings for discussion at ARC meetings. This will allow the ARC to include new models into its repertoire of rodent models with arterial stiffness. Other sessions will be devoted to methods being developed by ARC investigators. In addition, 6 seminar speakers are anticipated in the first year. Annual reports will include newly established collaborations, publications and project development by ARC investigators on the subject of stiffness.
Figures: