Ladan Amin Receives Alzheimer’s Fellowship
 Congratulations to Ladan Amin, a Postdoctoral Associate in the Harris laboratory, who was just awarded a three-year research fellowship from the Alzheimer’s Association. The title of her project is: “A new approach to visualize the interaction between Aβ and its receptors”.
Congratulations to Ladan Amin, a Postdoctoral Associate in the Harris laboratory, who was just awarded a three-year research fellowship from the Alzheimer’s Association. The title of her project is: “A new approach to visualize the interaction between Aβ and its receptors”.
Barbara Schreiber Appointed as Assistant Dean
 Dr. Schreiber has recently been appointed as Assistant Dean GMS Alumni Affairs.
Dr. Schreiber has recently been appointed as Assistant Dean GMS Alumni Affairs.
As the Assistant Dean for GMS Alumni Affairs, Dr. Schreiber will be responsible for:
- Serving as a liaison between GMS and the BUSM Office of Alumni Affairs representing GMS students and alumni in outreach efforts
- Coordinating and attending GMS alumni events and BUSM-wide events that include GMS alumni with the BUSM alumni office staff and deans
- Helping to identify GMS and other BUSM PhD alumni candidates for an annual award
- Developing a comprehensive database for PhD alumni
- Enabling a PhD alumni network that can offer support for doctoral and post-doctoral trainees in their professional development
Dr. Schreiber came to BUSM in 1975 as a graduate student in Microbiology and earned her PhD in Microbiology in 1981. She was a post-doctoral trainee in Clinical Microbiology at University Hospital from 1981-83 and a postdoctoral fellow in Biochemistry from 1983-87. Dr. Schreiber was appointed research assistant professor in 1987, research associate professor in 1997 and associate professor in 1999.
Dr. Schreiber currently serves as director of Biochemistry graduate studies, director of the GMS Program in Biomedical Sciences (PiBS), and course manager of the biochemistry class taught to first-year BU dental students and Oral Health Sciences students. Additionally, Dr. Schreiber is one of the co-PIs on the NIH-funded BU’s BEST award, dedicated to broadening experiences in biomedical science training for our PhD students and post-doctoral trainees.
Please join us in congratulating Dr. Schreiber on her latest appointment as Associate Dean, GMS Alumni Affairs.
Dr. Cathy Costello honored with special issue in The Journal of the American Society for Mass Spectrometry

In recognition of Dr. Catherine Costello's 2017 ASMS Award for a Distinguished Contribution in Mass Spectrometry, the entire June 2018 issue focused on Mass Spectrometry in Glycobiology and Related Fields and was a tribute to Dr. Costello's numerous contributions to the field. The first article in this issue written by Joe Zaia and Veronica Bierbaum describes Dr. Costello's vision and leadership in glycoscience mass spectrometry.
Please also read about the current research in the Costello lab.
New Software Developed for Glycosaminoglycan Analysis
The Zaia laboratory's recent study: “Software for peak finding and elemental composition assignment for glycosaminoglycan tandem mass spectra” has been selected to appear in a special virtual issue of ASBMB journal content on Omics of lipids, glycans and polar metabolites. This paper was selected from hundreds of papers and best represents the exciting advances made in studying these systems over the last three years.
Glycosaminoglycans (GAGs) covalently linked to proteoglycans are characterized by repeating disaccharide units and variable sulfation patterns along the chain. The Zaia laboratory and others have demonstrated the usefulness of tandem mass spectrometry (MS2) for assigning the structures of GAG saccharides; however, manual interpretation of tandem mass spectra is time-consuming, so computational methods must be employed. The Zaia group developed GAGfinder, the first tandem mass spectrum peak finding algorithm developed specifically for GAGs. GAGfinder is a targeted, brute force approach to spectrum analysis that utilizes precursor composition information to generate all theoretical fragments. GAGfinder also performs peak isotope composition annotation, which is typically a subsequent step for averagine-based methods.
Valentina Perissi Promotion
The Department would like to congratulate Dr. Valentina Perissi on her recent promotion to Associate Professor of Biochemistry. This is a well-deserved honor. Please read more about the ongoing research in the Perissi lab.
Professor of Biochemistry. This is a well-deserved honor. Please read more about the ongoing research in the Perissi lab.
Research Discoveries: Jello in Your Fat
A new study from the Layne lab "Aortic carboxypeptidase-like protein enhances adipose tissue stromal progenitor differentiation into myofibroblasts and is upregulated in fibrotic white adipose tissue " was recently published in PLoS One. This research identified a new pathway regulating of adipose tissue differentiation and fibrotic remodeling. First author and graduate student Mike Jager, along with collaborators in the Farmer and Fried labs determimed that the secreted protein Aortic Carboxypeptidase-like Protein (ACLP) repressed the differentiation of mouse and human adipocyte progenitors and was upregulated in fibrotic adipose tissue. ACLP was co-expressed with collagens in the adipose tissue and indicate that ACLP is a novel regulator of adipose progenitors and fibrosis.
" was recently published in PLoS One. This research identified a new pathway regulating of adipose tissue differentiation and fibrotic remodeling. First author and graduate student Mike Jager, along with collaborators in the Farmer and Fried labs determimed that the secreted protein Aortic Carboxypeptidase-like Protein (ACLP) repressed the differentiation of mouse and human adipocyte progenitors and was upregulated in fibrotic adipose tissue. ACLP was co-expressed with collagens in the adipose tissue and indicate that ACLP is a novel regulator of adipose progenitors and fibrosis.
Matthew Layne receives GMS Faculty Recognition Award
The winner of the 2018 Graduate Medical Sciences Faculty Recognition Award was announced yesterday at John McCahan Medical Education Day (May 30, 2018) . A faculty member who epitomizes this award that recognizes those who go above and beyond expectations for our teaching mission, our own Dr. Matthew Layne is this year’s honoree! Dr. Layne is a premier educator and one so very deserving of this honor! Congratulations Dr. Layne!
New research discoveries: glutamine metabolism controls breast cancer
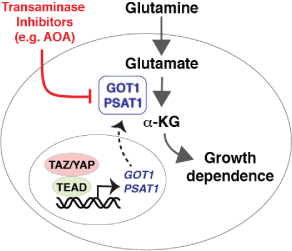 A new study from Dr. Varelas’ group has revealed important insights into the molecular mechanisms that control breast cancer cell growth. Their report, recently published in EMBO Reports, describes a role for the transcriptional regulators TAZ and YAP in driving oncogenic growth by promoting the expression of enzymes that control glutamine metabolism. Aggressive breast cancer cells are known to rely on an exogenous source of the amino acid glutamine for efficient growth. This study shows that TAZ and YAP contribute to glutamine dependence and reveals that cells with increased TAZ/YAP activity are vulnerable to compounds that inhibit key glutamine-utilizing enzymes, such as transaminases. Assessing TAZ/YAP activity in breast cancers may therefore offer a means for predicting response to targeted therapies with transaminase inhibitors, representing an exciting avenue for cancer therapy.
A new study from Dr. Varelas’ group has revealed important insights into the molecular mechanisms that control breast cancer cell growth. Their report, recently published in EMBO Reports, describes a role for the transcriptional regulators TAZ and YAP in driving oncogenic growth by promoting the expression of enzymes that control glutamine metabolism. Aggressive breast cancer cells are known to rely on an exogenous source of the amino acid glutamine for efficient growth. This study shows that TAZ and YAP contribute to glutamine dependence and reveals that cells with increased TAZ/YAP activity are vulnerable to compounds that inhibit key glutamine-utilizing enzymes, such as transaminases. Assessing TAZ/YAP activity in breast cancers may therefore offer a means for predicting response to targeted therapies with transaminase inhibitors, representing an exciting avenue for cancer therapy.
Research discoveries: new genetic form of connective tissue disease
A multi-laboratory collaboration has lead to the identification of genetic changes in the human AEBP1 gene, which encodes the Aortic Carboxypeptidase-like Protein (ACLP), that leads to defective collagen assembly and a variant of Ehlers Danlos Syndrome (EDS). This research was published in the American Journal of Human Genetics.
Researchers from the Layne laboratory, including students Rose Zhao, Kathleen Tumelty, and William Monis, along with researchers from the Mayo Clinic, King Faisal Hospital in Saudi Arabia, and at the Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health have identified families with AEBP1 genetic mutations resulting in a constellation of clinical findings including joint laxity, redundant and hyperextensible skin, poor wound healing with abnormal scarring, osteoporosis, and other features reminiscent of EDS. This study also showed that the ACLP enhances collagen polymerization and binds to several fibrillar collagens via its discoidin domain. These studies support the conclusion that biallelic pathogenic variants in AEBP1 are the cause of this autosomal recessive EDS subtype.
Blackburn PR, Xu Z, Tumelty KE, Zhao RW, Monis WJ, Harris KG, Gass JM, Cousin MA, Boczek NJ, Mitkov MV, Cappel MA, Francomano CA, Parisi JE, Klee EW, Faqeih E, Alkuraya FS, Layne MD, McDonnell NB, Atwal PS. Biallelic Alterations in AEBP1 Lead to Defective Collagen Assembly and Connective Tissue Structure Resulting in a Variant of Ehlers-Danlos Syndrome. Am J Human Genetics 2018, in press.
Research discoveries: glycoprotein glycosylation
New research papers from the Zaia laboratory have established novel strategies for analyzing glycans and glycopeptides.
Glycomics and glycoproteomics analyses by mass spectrometry require efficient front-end separation methods to enable deep characterization of heterogeneous glycoform populations. Chromatography methods a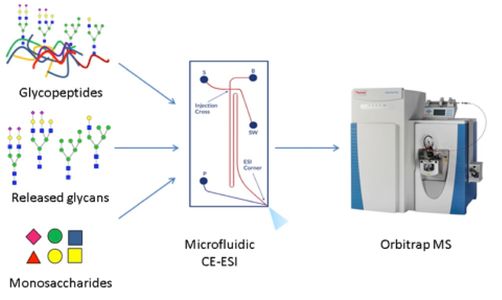 re generally limited in their ability to resolve glycoforms using mobile phases that are compatible with online liquid chromatography-mass spectrometry (LC-MS). The adoption of capillary electrophoresis-mass spectrometry methods (CE-MS) for glycomics and glycoproteomics is limited by the lack of convenient interfaces for coupling the CE devices to mass spectrometers. Here, we describe the application of a microfluidics-based CE-MS system for analysis of released glycans, glycopeptides and monosaccharides. We demonstrate a single CE method for analysis three different modalities, thus contributing to comprehensive glycoproteomics analyses. In addition, we explored compatible sample derivatization methods. We used glycan TMT-labeling to improve electrophoretic migration and enable multiplexed quantitation by tandem MS. We used sialic acid linkage-specific derivatization methods to improve separation and the level of information obtained from a single analytical step. Capillary electrophoresis greatly improved glycoform separation for both released glycans and glycopeptides over that reported for chromatography modes more frequently employed for such analyses. Overall, the CE-MS method described here enables rapid setup and analysis of glycans and glycopeptides using mass spectrometry
re generally limited in their ability to resolve glycoforms using mobile phases that are compatible with online liquid chromatography-mass spectrometry (LC-MS). The adoption of capillary electrophoresis-mass spectrometry methods (CE-MS) for glycomics and glycoproteomics is limited by the lack of convenient interfaces for coupling the CE devices to mass spectrometers. Here, we describe the application of a microfluidics-based CE-MS system for analysis of released glycans, glycopeptides and monosaccharides. We demonstrate a single CE method for analysis three different modalities, thus contributing to comprehensive glycoproteomics analyses. In addition, we explored compatible sample derivatization methods. We used glycan TMT-labeling to improve electrophoretic migration and enable multiplexed quantitation by tandem MS. We used sialic acid linkage-specific derivatization methods to improve separation and the level of information obtained from a single analytical step. Capillary electrophoresis greatly improved glycoform separation for both released glycans and glycopeptides over that reported for chromatography modes more frequently employed for such analyses. Overall, the CE-MS method described here enables rapid setup and analysis of glycans and glycopeptides using mass spectrometry
To learn more:
- Khatri, K.; Klein, J. A.; Zaia, J. Use of an informed search space maximizes confidence of site-specific assignment of glycoprotein glycosylation. Anal Bioanal Chem 2017, 409, 607-618. Pubmed Link
- Khatri, K.; Klein, J. A.; Haserick, J.; Leon, D. R.; Costello, C. E.; McComb, M. E.; Zaia, J. Microfluidic capillary electrophoresis-mass spectrometry for analysis of monosaccharides, oligosaccharides and glycopeptides. Anal. Chem. 2017, 89, 6645-6655. Pubmed Link