Congratulations to David A. Harris, M.D.,Ph.D. on being awarded The Edgar Minas Housepian, MD, Professorship
Congratultions to David A. Harris on being one of 5 recipients of the Edward Avedisian Professorships.

The Edgar Minas Housepian, MD, Professorship went to David A. Harris, chair of biochemistry andcell biology since 2009. He studies molecular and cellular mechanisms underlying human neurodegenerative diseases. His work on infectious prion diseases like mad cow disease, where brain proteins fold and can result in neurodegenerative effects, has helped research into other neurodegenerative diseases, like Alzheimer’s, Parkinson’s, and Huntington’s.
“What I want to highlight here is the incredible foresight to use that endowment ($25 million of the Avedisian endowment is dedicated to research and teaching) to support basic research, which is…always at the root of great medical discoveries,” said Harris. “I am honored to be associated with a legacy that values the pursuit of knowledge and scientific excellence.”
Housepian was a renowned neurosurgeon at New York-Presbyterian Hospital and professor of neurology at Columbia University’s medical school, where he taught for 44 years.
“He was a very creative person with a long-range vision,” said his daughter, Jean Housepian. Even with a career that began in labs, then surgery, education remained his key concern, and in his retirement years, he remained an advocate for international educational affiliations for medical students.
See the whole article here.
Robert Mercer recently awarded a research grant funded by Creutzfeldt-Jakob Disease Foundation.
 Robert Mercer, an Instructor in the Harris lab, was recently awarded a research grant funded by Creutzfeldt-Jakob Disease Foundation. This two-year, $100,000 grant is entitled “Microenvironment mapping of the PrPSc Interactome”.
Robert Mercer, an Instructor in the Harris lab, was recently awarded a research grant funded by Creutzfeldt-Jakob Disease Foundation. This two-year, $100,000 grant is entitled “Microenvironment mapping of the PrPSc Interactome”.
Prion diseases are fatal and currently untreatable neurodegenerative diseases of humans and animals. The central event underlying these diseases is the 3D conversion of the prion protein (PrPC) to a pathogenic shape, denoted PrPSc. In attempts to elucidate its function, there have been multiple studies analyzing the interaction network, or interactome, of PrPC. However, almost nothing is known about the interaction network of PrPSc. Here, we propose to examine the PrPSc interactome for the first time, providing a wealth of new targets for therapeutic intervention.
Grant Award: Department of Defense, Discovery Award

Robert Mercer, a Postdoctoral Associate in the Harris Lab, was recently awarded a Discovery Award research grant funded by the Department of Defense. This two-year, $200,000 grant is entitled “ Identification of Receptors for TAR DNA-binding Protein 43 (TDP-43) That Mediate Cellular Uptake and Neurotoxicity ”. This project aims to identify the cellular receptors of the pathological TDP-43 assemblies found in the brains of patients with Amyotrophic Lateral Sclerosis (ALS), Frontotemporal Lobar Degeneration (FTLD), and Chronic Traumatic Encephalopathy (CTE). The identification of these receptors will provide novel therapeutic targets to combat these devastating and currently untreatable diseases.
Grant Awards: Visualizing Alzheimer’s Disease
 Ladan Amin an Instructor in the Harris laboratory was recently awarded a R03 research grant, funded by the National Institute on Aging (NIA). This two-year, $200,000 grant is entitled “Use of super-resolution microscopy to visualize the interaction between Alzheimer therapeutic antibodies and Aβ aggregates”. This project aims to identify the molecular mechanism of action of several different clinical antibodies by localizing them on single Aβ aggregates. This information will contribute significantly to understanding the basic biology of amyloid and its role in Alzheimer’s disease, and will provide a fuller picture of molecular mechanisms responsible for detoxifying oligomers and enhancing their clearance.
Ladan Amin an Instructor in the Harris laboratory was recently awarded a R03 research grant, funded by the National Institute on Aging (NIA). This two-year, $200,000 grant is entitled “Use of super-resolution microscopy to visualize the interaction between Alzheimer therapeutic antibodies and Aβ aggregates”. This project aims to identify the molecular mechanism of action of several different clinical antibodies by localizing them on single Aβ aggregates. This information will contribute significantly to understanding the basic biology of amyloid and its role in Alzheimer’s disease, and will provide a fuller picture of molecular mechanisms responsible for detoxifying oligomers and enhancing their clearance.
Nhat Le receives Warren Alpert Distinguished Scholars Award
 Congratulations to Dr. Nhat Le, a Postdoctoral Research Associate in the Harris lab, who was recently awarded a Warren Alpert Distinguished Scholars career development award. This two-year, $400,000 fellowship will fund Nhat’s work on a project entitled “Signaling Pathways Underlying Prion Neurotoxicity”. Her project aims to identify the mechanism underlying prion-induced synaptotoxicity using genomic, proteomic and pharmacological techniques in mouse and human neurons and in animal models.
Congratulations to Dr. Nhat Le, a Postdoctoral Research Associate in the Harris lab, who was recently awarded a Warren Alpert Distinguished Scholars career development award. This two-year, $400,000 fellowship will fund Nhat’s work on a project entitled “Signaling Pathways Underlying Prion Neurotoxicity”. Her project aims to identify the mechanism underlying prion-induced synaptotoxicity using genomic, proteomic and pharmacological techniques in mouse and human neurons and in animal models.
New Research Discoveries: Prions and Synaptic Toxicity
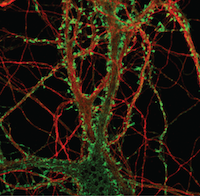
 In a new research study from the Harris laboratory in PLOS pathogens, first author Cheng Feng and collaborators defined a new pathway mediated by the p38 MAPK signaling pathway that was important for prion synaptic toxicity. Furthermore this research identified that perturbations in the actin cytoskeleton within dendritic spines resulted in deficits in synaptic transmission.
In a new research study from the Harris laboratory in PLOS pathogens, first author Cheng Feng and collaborators defined a new pathway mediated by the p38 MAPK signaling pathway that was important for prion synaptic toxicity. Furthermore this research identified that perturbations in the actin cytoskeleton within dendritic spines resulted in deficits in synaptic transmission.
This study has received significant press coverage and appeared on the cover of PLOS pathogens.
Congratulations Harris lab.
Ladan Amin Receives Alzheimer’s Fellowship
 Congratulations to Ladan Amin, a Postdoctoral Associate in the Harris laboratory, who was just awarded a three-year research fellowship from the Alzheimer’s Association. The title of her project is: “A new approach to visualize the interaction between Aβ and its receptors”.
Congratulations to Ladan Amin, a Postdoctoral Associate in the Harris laboratory, who was just awarded a three-year research fellowship from the Alzheimer’s Association. The title of her project is: “A new approach to visualize the interaction between Aβ and its receptors”.
New Research Discoveries: Prion proteins and neuron degeneration
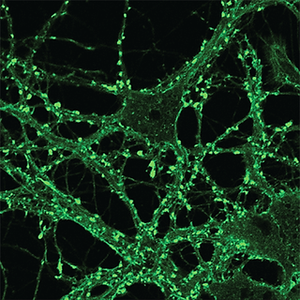
A new paper in eLife from the Harris lab has uncovered a novel function for different domains of the prion protein. Bei Wu, an Instructor in the laboratory was lead author.
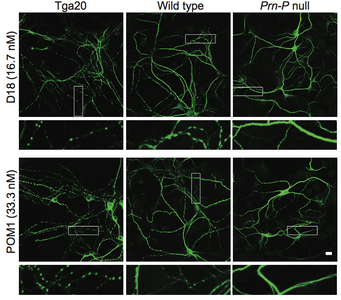
Prion diseases, or transmissible spongiform encephalopathies, comprise a group of fatal neurodegenerative disorders in humans and animals for which there are no effective treatments or cures. These diseases are caused by refolding of the cellular prion protein (PrPC) into an infectious isoform (PrPSc) that catalytically templates its abnormal conformation onto additional molecules of PrPC.A similar, prion-like process may play a role in other neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases and tauopathies, which are due to protein misfolding and aggregation. Here, using a combination of electrophysiological, cellular, and biophysical techniques, we show that the flexible, N-terminal domain of PrPC functions as a powerful toxicity-transducing effector whose activity is tightly regulated in cis by the globular C-terminal domain. Ligands binding to the N-terminal domain abolish the spontaneous ionic currents associated with neurotoxic mutants of PrP, and the isolated N-terminal domain induces currents when expressed in the absence of the C-terminal domain. Anti-PrP antibodies targeting epitopes in the C-terminal domain induce currents, and cause degeneration of dendrites on murine hippocampal neurons, effects that entirely dependent on the effector function of the N-terminus. NMR experiments demonstrate intramolecular docking between N- and C-terminal domains of PrPC, revealing a novel auto-inhibitory mechanism that regulates the functional activity of PrPC.
New Research Discoveries—Prion Synaptotoxicity
Congratulations to Cheng Fang and colleagues in the Harris lab for their recent paper in PLOS Pathogens which developed an in vitro neuronal system in which to study the early assault by prions on brain cells of the infected host. This system provides new insights into the mechanisms responsible for prion neurotoxicity, and it provides a platform for characterizing different pathogenic forms of PrPSc and testing potential therapeutic agents.
Congratulations to Cheng Fang in the Harris Lab
Congratulations to Dr. Cheng Fang in the Harris laboratory for winning first prize at the "Prion 2015" meeting in Colorado for her poster presentation "PrPSc-containing brain samples induce PrPC-dependent retraction of dendritic spines in primary neurons".