Research Focus
“Rewarding science is born out of creativity, tenacity and generosity.”
(Katya Ravid)
Overview: Our lab is conducting investigations in the area of blood and vascular pathologies. The cells of all blood lineages arise from pluripotent hematopoietic stem cells that reside in the marrow. The bone marrow also contains stem cells of other lineages, including fat, vascular etc. Our research is focused on two interrelated projects that bear on mechanisms associated with the development of blood and vascular pathologies: (1) molecular mechanisms involved in bone marrow megakaryocyte/ platelet development; (2) the role of vascular and bone marrow cell (mesenchymal stem cells) adenosine receptors in tissue regeneration. Transgenic and knockout mouse models are used to assist in exploring mechanisms in vivo.
A. New center led by Dr. Ravid to examine the link between cancer (including of hematopoietic origin) and cardiovascular diseases, with a special focus on understanding and preventing health disparities. Please see:
Why Are Black Cancer Patients at Higher Risk for Blood Clots?
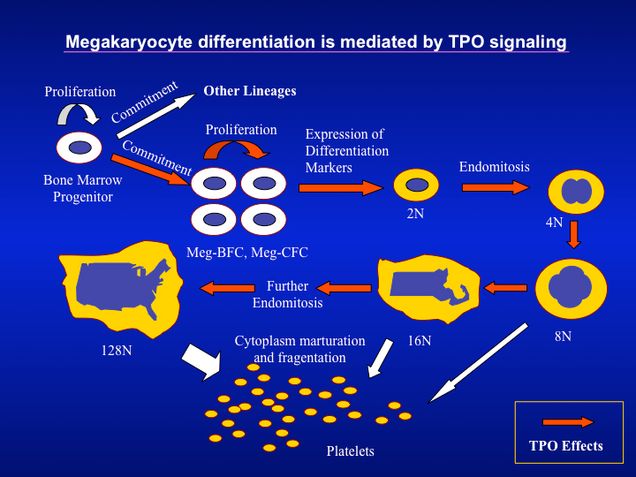
B. The cells of all blood lineages arise from pluripotent hematopoietic stem cells. The mechanisms that regulate lineage commitment and the subsequent steps of cellular maturation are not fully understood. In the megakaryocytic lineage, the early committed cells undergo a unique cell cycle (endomitosis) leading to the formation of polyploid cells prior to fragmenting into platelets. The lab’s goal has been to study the genetic and signaling factors that control megakaryocyte/ platelet development, with a focus on the endomitotic process. Our group was the first to describe the important role of D-type cyclins in promoting endomitotis in megakaryocytes in vivo. Studies in cell lines and in vivo pointed to the importance of investigating this process in primary cells or in vivo. In a recent study, megakaryocytes were labeled in vivo at the chromosome level with histone-GFP targeted via the PF4 promoter, yielding live images of the endomitotic process in this lineage. Understanding mechanisms of polyploidy is not only relevant to this lineage, but to other cell types that are known to become polyploidy under stress. For instance, our group was the first to identify vascular smooth muscle polyploidy as a biomarker for aging and to report the mechanism of control of the endomitotic process in these smooth muscle cells, including by live imaging.
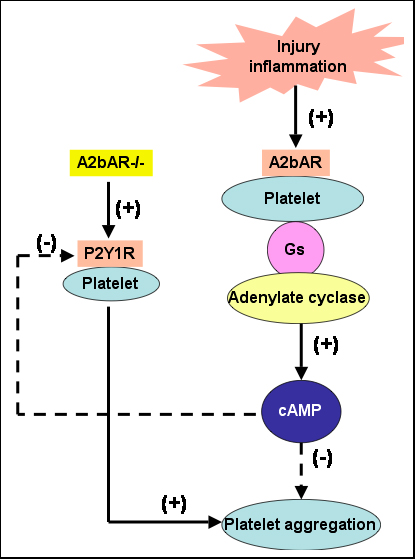
C. The study of the role of adenosine receptors pointed to the importance of these receptors expression in bone marrow cells and vascular smooth muscle cells for tissue regeneration and vascular response to injury and atherosclerosis. We generated an A2B adenosine receptor-knockout/reporter gene-knock-in (A2BAR-knockout/reporter gene-knock-in) mouse model and showed receptor gene expression in the vasculature and macrophages, the ablation of which causes low-grade inflammation compared with age-, sex-, and strain-matched control mice. Augmentation of proinflammatory cytokines, such as TNF-alpha, and a consequent downregulation of IkappaB-alpha are the underlying mechanisms for an observed upregulation of adhesion molecules in the vasculature of these A2BAR-null mice. Intriguingly, leukocyte adhesion to the vasculature is significantly increased in the A2BAR-knockout mice. Exposure to an endotoxin results in augmented proinflammatory cytokine levels in A2BAR-null mice compared with control mice. Bone marrow transplantations indicated that bone marrow (and to a lesser extent vascular) A2BARs regulate these processes. Hence, we identify the A2BAR as a new critical regulator of inflammation and vascular adhesion primarily via signals from hematopoietic cells to the vasculature, focusing attention on the receptor as a therapeutic target. Ongoing studies are focusing on the role of adenosine receptors in the differentiation of mesenchymal stem cells into different lineages. To view other experimental models please click here.