The Zoeller Lab
Research
Forward Genetics: Animal Cell Lines Defective in Lipid Biosynthesis
Lipids are not only structural units of membranes. They also participate in important cellular functions, serving as second messengers, hormones, pheromones and membrane anchors for proteins. Although several “active” lipid species have been identified, there are many others that remain undiscovered. To identify functional roles for lipids, we’ve developed mutant animal cell lines that are deficient in the biosynthesis and/or transport of specific lipid species (forward genetics). To date, our laboratory has isolated ten unique cell lines with defects in different aspects of animal cell lipid metabolism, biosynthesis and transport (references 1-8). Using these mutants, we can determine what cellular processes are affected by the loss of the lipid, establishing a role for the lipid in that process, and examine the metabolism of the lipid(s). The mutants can also serve as tools for the isolation of the genes involved in their biosynthesis, metabolism or transport. Most importantly, the study of these mutants leads to new biochemistry, not achievable through conventional approaches.
A New Direction: Antiviral Therapies, High-Throughput Screening for Small Molecule Inhibitors of Animal Cell Phospholipid Biosynthesis
Viral infection results in both acute and chronic pathologies encompassing a wide range of symptoms. As an example, influenza virus affects three to five million people and results in 250,000 to 500,000 deaths every year, worldwide. These numbers rise dramatically during pandemics. Effective preventative protocols do not exist for many viral pathogens and there are few successful post-infection treatments.
Generation of new membranes or lipid structures in host cells is required for viral replication of many pathogenic viruses including hepatitis C, poliovirus, HIV and influenza. Viruses rely heavily on host biosynthesis of phospholipids. Importantly, inhibition of this process has been shown to prevent viral replication. We feel that compounds targeting animal cell phospholipid biosynthesis would be effective antiviral agents. This approach is attractive because, while mutations within the viral DNA are likely to produce drug resistance against compounds that target viral structures or processes, compounds that target a host process such as phospholipid biosynthesis, critical to viral replication, would greatly reduce the likelihood of development of resistant viral strains. Also, these compounds would be broad-spectrum antiviral agents, not specific for a single viral strain. To date, there have been no high-throughput screening (HTS) protocols available to screen the large number of available compound libraries for inhibitors of animal cell phospholipid biosynthesis. We have recently developed a cell-based HTS that can be used for this purpose (reference 9).
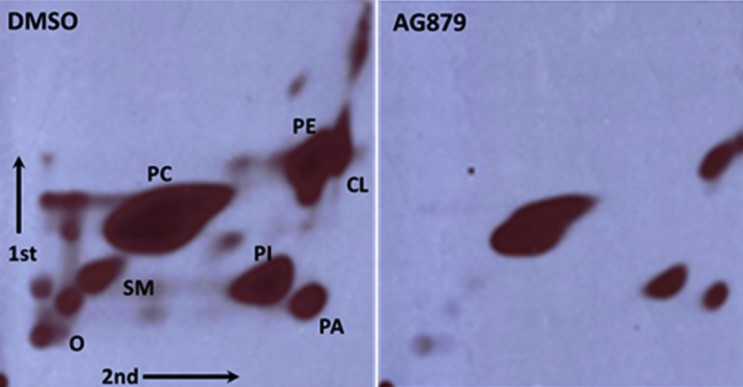
Using this initial protocol, we identified and reported on a compound, tyrphostin AG879, that inhibits phospholipid biosynthesis in animal cell by 80-85% at 10 µM with an EC50 of 1-3 µM (Figure 1). Since then, we have refined and improved the screening protocol. Using the new protocol, we have identified an additional four compounds that also inhibit this process. Importantly, all five compounds we have identified to date have been shown, independent of our laboratory, to have antiviral properties. The availability of such inhibitors will not only be useful in antiviral therapies, but will also yield valuable information about the factors that influence phospholipid biosynthesis in mammalian cells. We are currently collaborating with investigators from the Boston University’s National Emerging Infectious Diseases Laboratories (NEIDL) to further examine antiviral capabilities of some of our phospholipid biosynthesis inhibitors in pathological viruses such as Ebola.
Relevent Publications:
1. Zoeller RA, Raetz CRH. 1986. Isolation of Animal Cell Mutants Deficient in Plasmalogen Biosynthesis and Peroxisome Assembly. Proc. Natl. Acad. Sci. 83:5170-5174.
2. James P, Lee J, Rizzo WB, Zoeller RA. 1990. Isolation and Characterization of a Chinese Hamster Ovary Cell Line Deficient in Fatty Alcohol: NAD+ Oxidoreductase. Proc. Natl. Acad. Sci. USA 87:6102-6106.
3. Zoeller RA, Rangaswamy S, Herscovitz H, Rizzo WB, Hajra AK, Das AK, Moser HW, Moser A, Lazarow PB, Santos MJ. 1992. Mutants in a Macrophage-like Cell Line are Defective in Plasmalogen Biosynthesis, but Contain Functional Peroxisomes. J. Biol. Chem. 267:8299-8306.
4. Zoeller RA, Layne M, & Modest, E. 1995. Animal Cell Mutants Unable to Take Up Biologically Active Phospholipids. J. Lipid Res. 36, 1866-1875.
5. James PF & Zoeller RA. 1997. Isolation of Animal Cell Mutants Defective in Long-Chain Fatty Aldehyde Dehydrogenase. Sensitivity to fatty aldehydes and Schiff’s base modification of phospholipids: Implications for Sjögren-Larsson Syndrome.” J. Biol. Chem., 272, 23540-23546.
6. James PF, Lake AC, Hajra AK, Larkins LK, Robinson M, Buchanan FG, & Zoeller RA. 1997. An Animal Cell Mutant with a Defect in Acyl/Alkyl-dihydroxyacetonephosphate Reductase. Effects on the biosynthesis of ether-linked and diacyl glycerolipids. J. Biol. Chem, 272, 23547-23553.
7. Nagan N, Hajra AK, Larkins LK, Lazarow P, Purdue PE, Rizzo WB, & Zoeller RA. 1998. Isolation of a Chinese hamster fibroblast variant defective in dihydroxyacetonephosphate acyltransferase activity and plasmalogen biosynthesis: use of a novel two-step selection protocol. Biochem J., 332:273-279.
8. Haller JF, Smith C, Liu D, Zheng H, Tornheim K, Han G, Carman GM & Zoeller RA. 2010. Isolation of novel animal cell linesdefective in glycerolipid biosynthesis reveals mutations in glucose-6-phosphate isomerase. J. Biol. Chem. 285:866-877.
9. Zoeller RA, Geoghegan-Barek K. 2019. A cell-based high-throughput screen identifies tyrphostin AG 879 as an inhibitor of animal cell phospholipid and fatty acid biosynthesis. Biochem Biophys Rep. 18, 100624 PMID: 30899803
Links:
BU Profile
ResearchGate
PubMed
Contact Us
Department of Pharmacology, Physiology & Biophysics
Chobanian & Avedisian School of Medicine
700 Albany Street, W308A
Boston MA 02118-2526
Phone:(617) 358-8752
e-mail: rzoeller@bu.edu