The Makino Lab
Research
The rate of cGMP synthesis modulates visual transduction
In retinal rods, cGMP is the second messenger that links photon capture by rhodopsin on internal disk membranes to ion channel activity on the plasma membrane. Rhodopsin photoexcitation leads to the hydrolysis of cGMP and subsequent closure of cGMP-gated channels, curtailing the entry of Na+ and Ca2+. During response recovery, retina-specific guanylyl cyclases (retGCs) replenish cGMP, reopen the channels, and restore the influx of cations. To facilitate the recovery, guanylyl cyclase activating proteins (GCAPs) sense the decrease in Ca2+ caused by illumination and greatly stimulate the rate of cGMP synthesis.
All vertebrate rods use at least two GCAPs: GCAP1 and GCAP2. Why are 2 types of GCAPS necessary? Working closely with the Dizhoor laboratory at Salus University, we explored the basis for the dual system by using electrophysiological methods to study rods of mutant mice that lack one or both GCAPs. Deletion of GCAP2 did not change the amplitude of the single photon response, but slowed its recovery (Fig. 1). Elimination of GCAP1 caused the photon response to rise for twice as long to an amplitude that was twice as large. Although knockout of GCAP2 did not affect GCAP1 expression, knockout of GCAP1 did cause an up-regulation of GCAP2 as detected by immunofluorescence and Western blot. The overexpression of GCAP2 resulted in acceleration of the response recovery rather than the slowdown that was expected from the loss of GCAP1 alone.
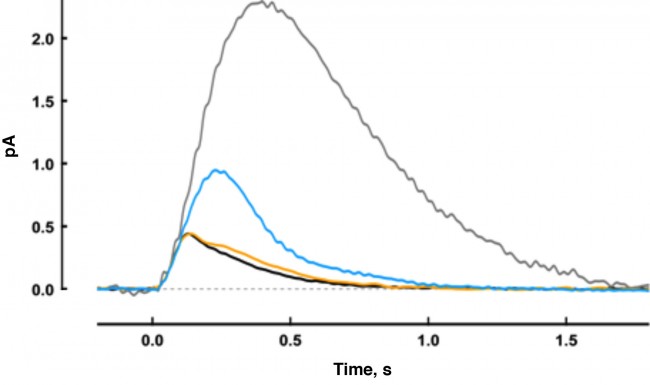
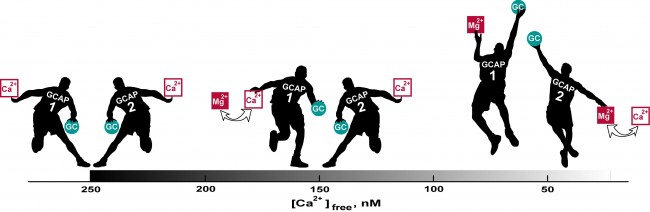
Thus the two GCAPs regulate retGCs sequentially (Fig. 2). Because of its lower Ca2+ affinity, GCAP1 is the first responder and acts to limit response amplitude. GCAP2, with a higher Ca2+ affinity, does not assist retGCs until Ca2+ concentration declines even further, which happens somewhat during a single photon response, but to a greater extent with bright light. GCAP2 stimulation of retGCs provides for a faster response recovery. Together, the two GCAPs tune the operating range and temporal resolution of rod photoreceptors.
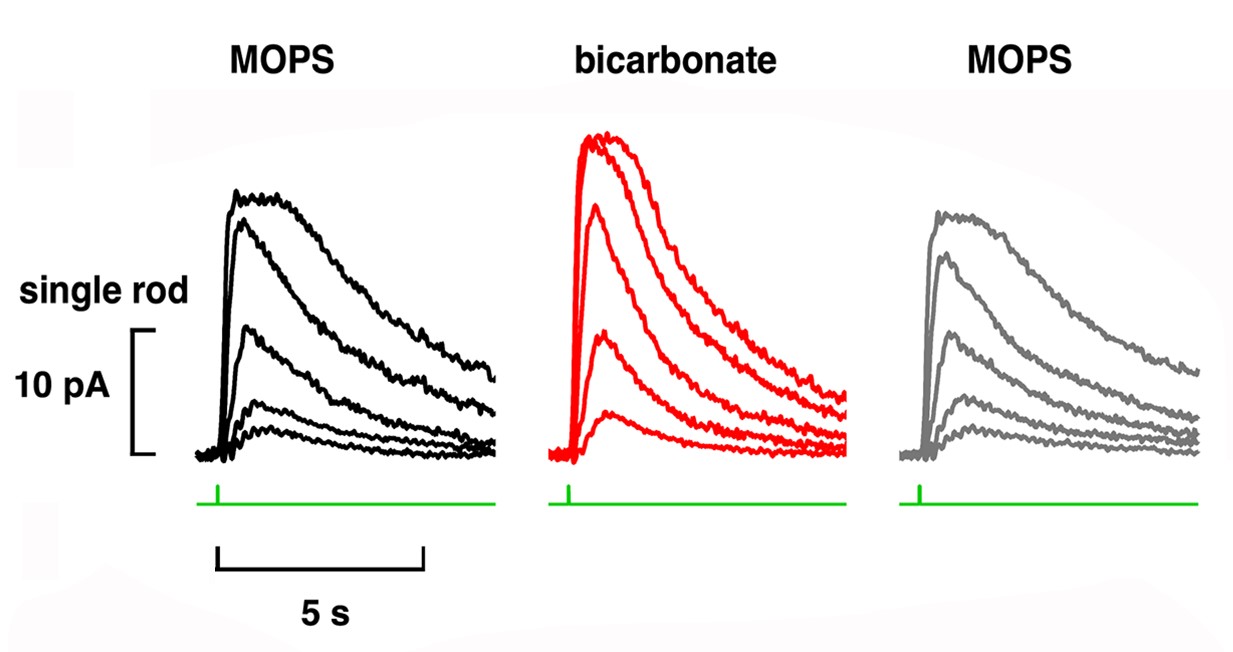
The Sharma and Duda laboratories at Salus University discovered that bicarbonate ions stimulate cGMP synthesis by retGC, independent of GCAPs. In single cell recordings, we discovered that bicarbonate operated synergistically with GCAPs and low Ca2+ in rods and in cones. As a result, bicarbonate boosted the size of the maximal flash response, reduced relative sensitivity to flashes and altered response kinetics (Fig. 3).
Rods do not express carbonic anhydrase, suggesting that modulation of visual transduction involves bicarbonate produced exogenously. We found that only rods retaining an intact spherule were affected by bicarbonate, indicating that uptake occurred in the inner segment, near the synaptic ending. In contrast, cones took up bicarbonate at their outer segments as well as at their inner segments. Moreover, cones did express carbonic anhydrase, which meant that they were affected by endogenous as well as exogenous bicarbonate.
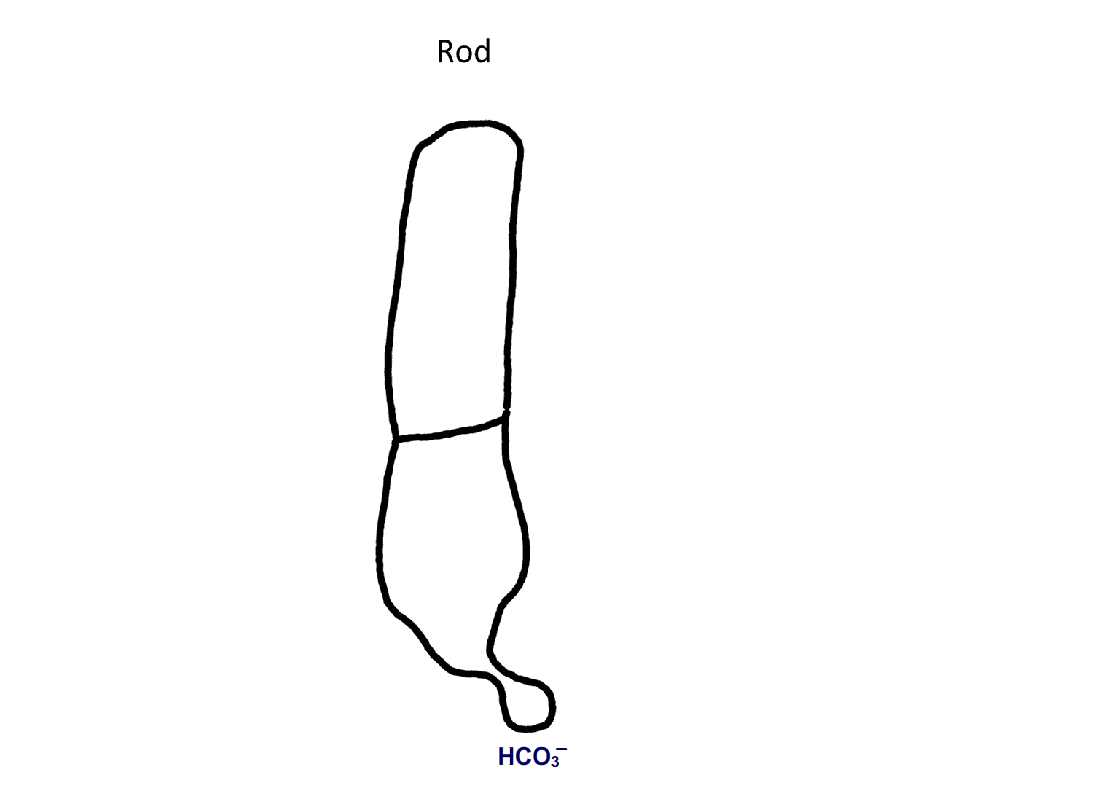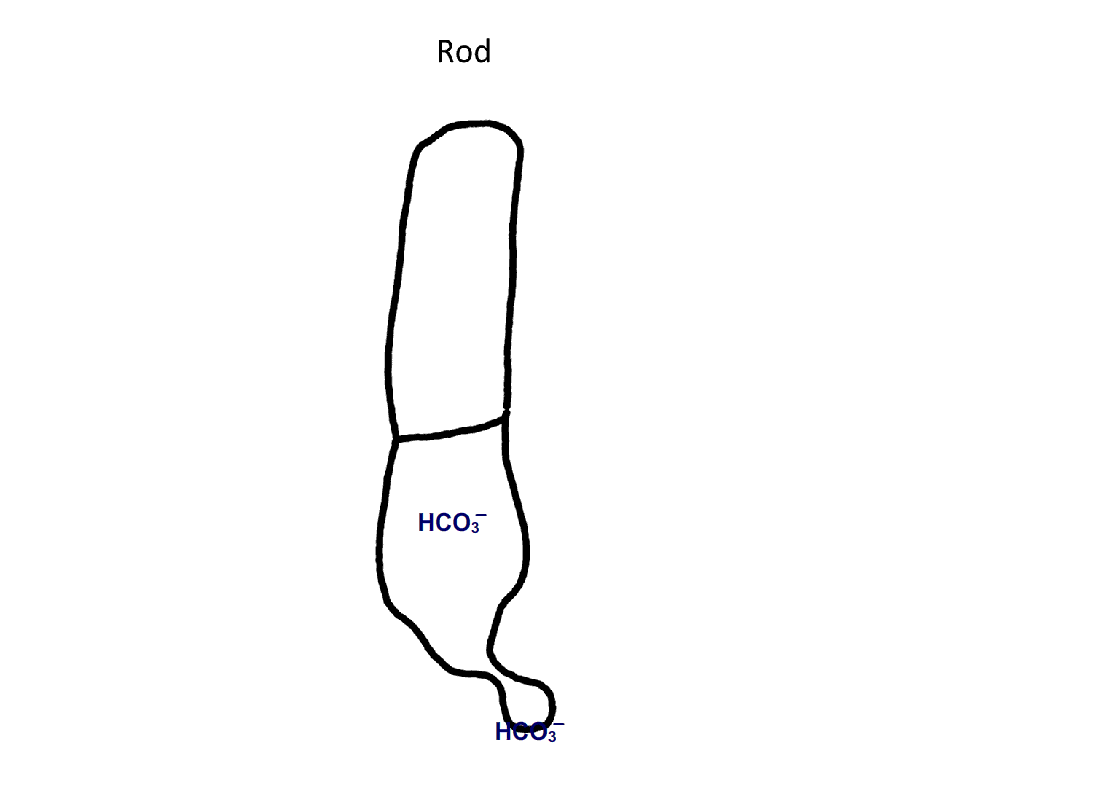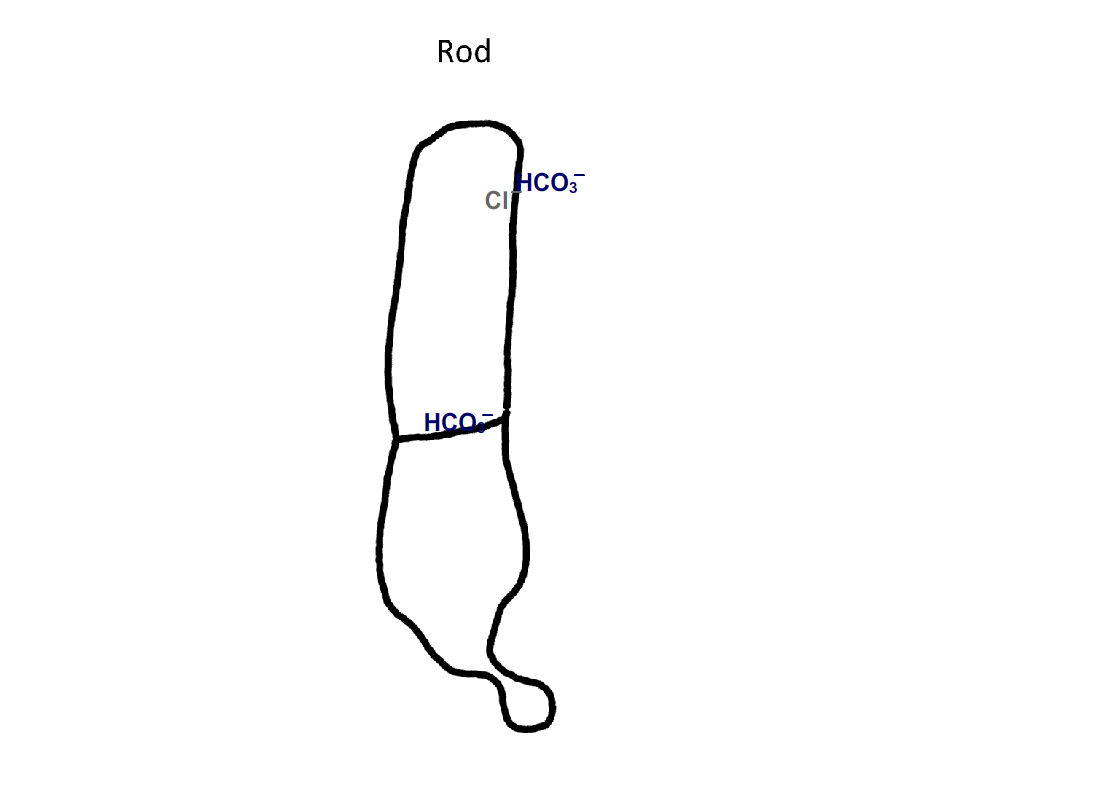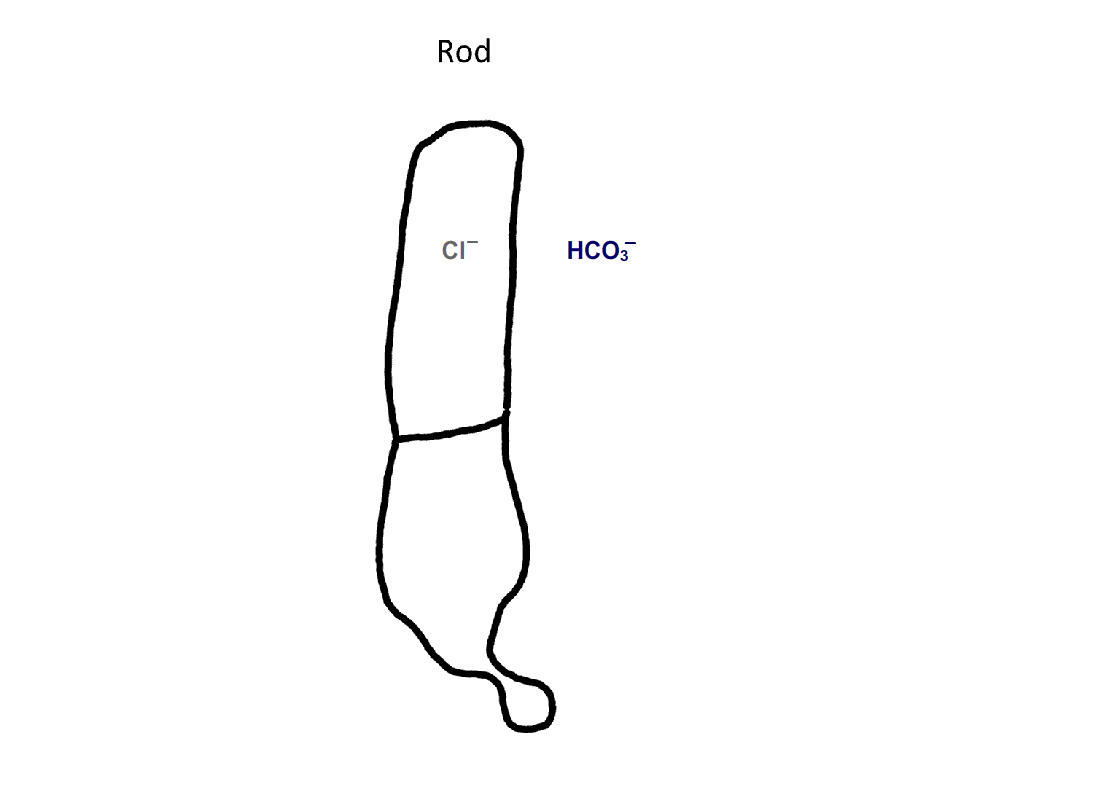
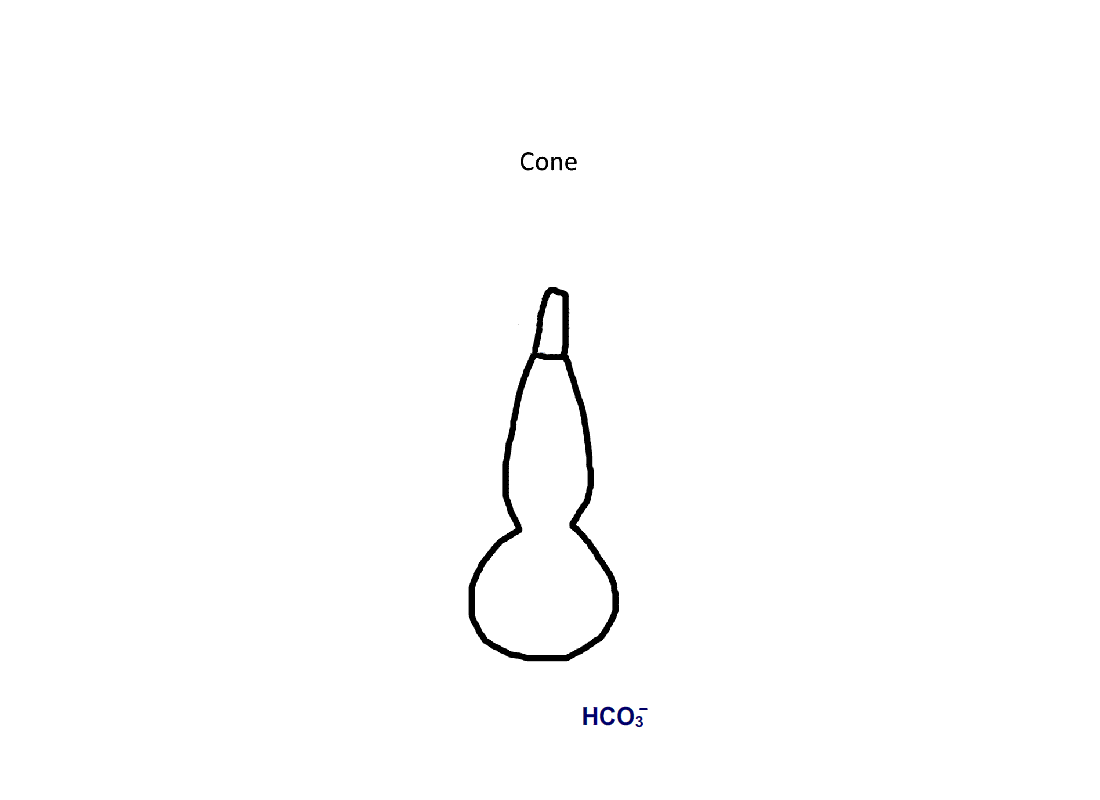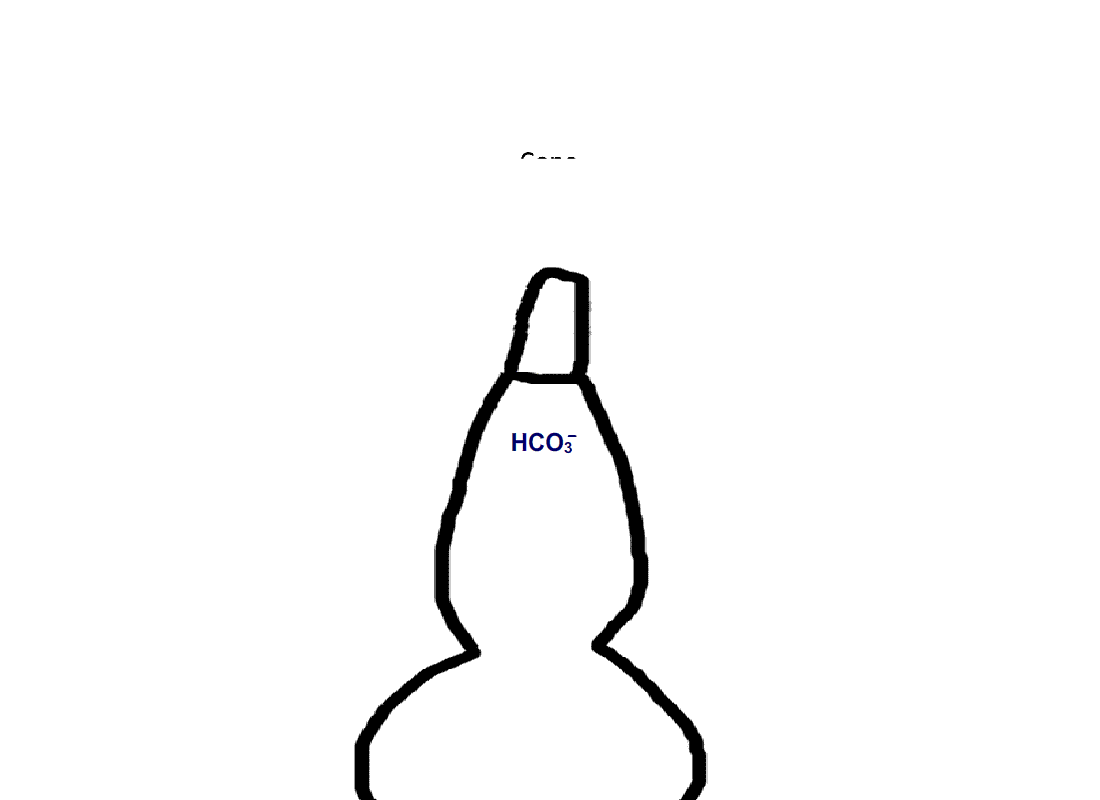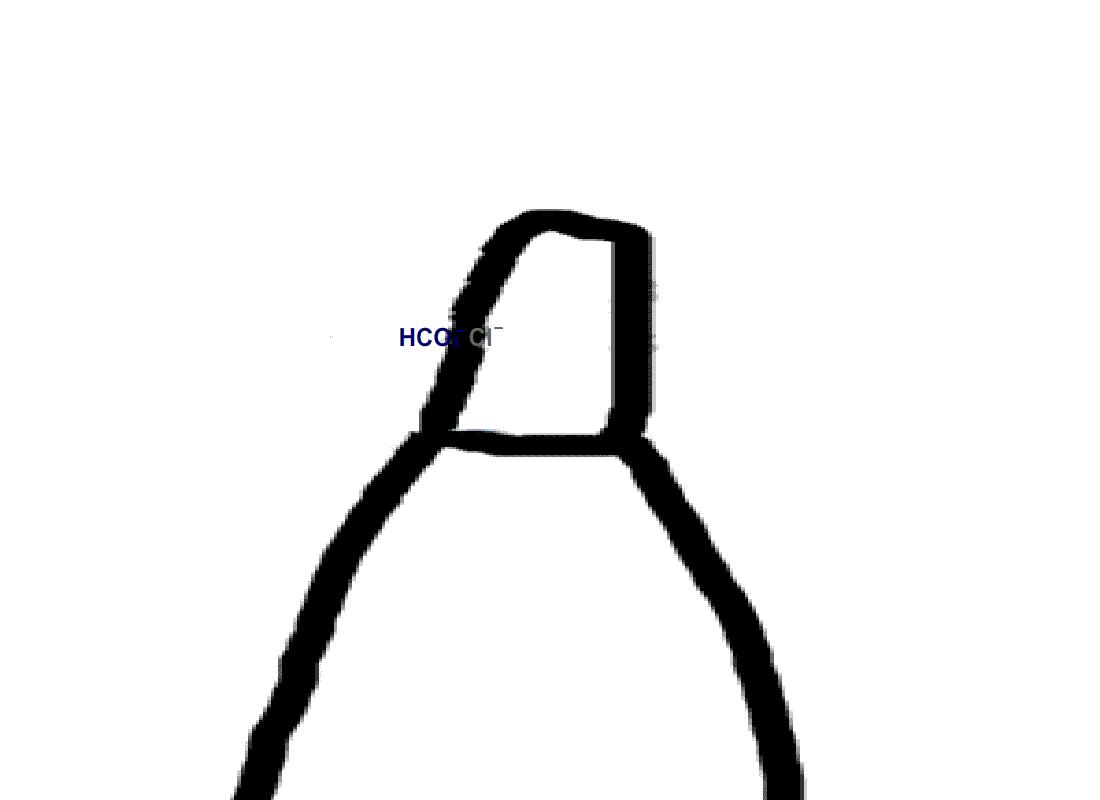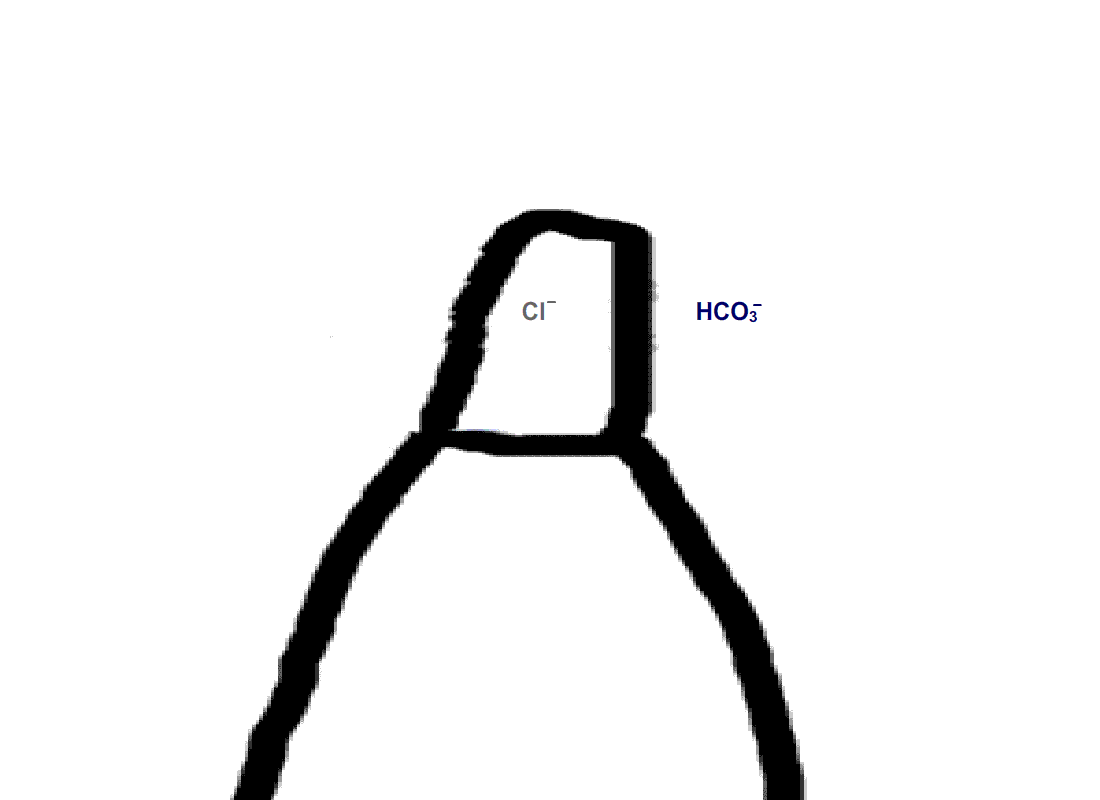
Figure 4. Pathway for bicarbonate through rods and cones. Bicarbonate enters rods and cones at their synapses (1). Cones are also able to take up bicarbonate at their outer segments, perhaps by reversed action of a bicarbonate/Cl- exchanger (2). Carbon dioxide is able to pass through the plasma membrane and in cones, but not in rods, gets converted to bicarbonate by carbonic anhydrase. Bicarbonate is removed from rods and cones by a bicarbonate/Cl- exchanger located in their outer segments.
Recent Publications
Full listing of citations on PubMed
PAPERS
Makino, C.L., Duda, T., Pertzev, A., Isayama, T., Geva, P., Sandberg, M.A. and Sharma, R.K. (2019) Modes of accessing bicarbonate for the regulation of membrane guanylate cyclase (ROS-GC) in retinal rods and cones. eNeuro, 6(1). PMCID: PMC6378327.
Duda, T., Pertzev, A., Makino, C.L. & Sharma, R.K. (2016) Bicarbonate and Ca2+ sensing modulators activate photoreceptor ROS-GC1 synergistically. Frontiers in Molecular Neuroscience 9: 5.
Duda, T., Wen, X.-H., Isayama, T., Sharma, R.K. and Makino, C.L. (2015) Bicarbonate modulates photoreceptor guanylate cyclase (ROS-GC) catalytic activity. Journal of Biological Chemistry 290: 11052-11060.
Isayama, T., Chen, Y., Kono, M., Fabre, E., Slavsky, M., DeGrip, W.J., Ma, J.-X., Crouch, R.K. and Makino, C.L. (2014) Co-expression of three opsins in cone photoreceptors of the salamander, Ambystoma tigrinum. Journal of Comparative Neurology 522: 2249-2265. PMCID: PMC3997598.
Makino, C.L., Wen, X.-H., Olshevskaya, E.V., Peshenko, I.V., Savchenko, A.B. and Dizhoor, A.M. (2012) Enzymatic relay mechanism stimulates cyclic GMP synthesis in rod photoresponse: biochemical and physiological study in guanylyl cyclase activating protein 1 knockout mice. PLoS ONE 7: e47637. PMCID: PMC3474714.
Makino, C.L., Wen, X.-H., Michaud, N.A., Covington, H.I., DiBenedetto, E., Hamm, H.E., Lem, J., and Caruso, G. (2012) Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS ONE 7: e37832. PMCID: PMC3360601.
Wen, X.-H., Duda, T., Pertzev, A., Venkataraman, V., Makino, C.L. and Sharma, R.K. (2012) S100B serves as a Ca2+ sensor for ROS-GC1 guanylate cyclase in cones but not in rods of the murine retina. Cellular Physiology and Biochemistry 29: 417-430. PMCID: PMC3434333.
REVIEWS
Sharma, R.K., Duda, T. and Makino, C.L. (2016) Integrative signaling networks of membrane guanylate cyclases: Biochemistry and physiology. Frontiers in Molecular Neuroscience 9: 83. doi: 10.3389/fnmol.2016.00083. PMCID: PMC5023690.
Wen, X.-H., Dizhoor, A.M. and Makino, C.L. (2014) Membrane guanylyl cyclase complexes shape the photoresponses of retinal rods and cones. Frontiers in Molecular Neuroscience 7:45. PMCID: PMC4040495.
Sharma, R.K., Makino, C.L., Hicks, D. and Duda, T. (2014) ROS-GC interlocked Ca2+-sensor S100B protein signaling in cone photoreceptors: review. Frontiers in Molecular Neuroscience 7: 21. PMCID: PMC3972482.
BOOK CHAPTERS
Makino, C.L,. Duda, T., Pertzev, A. and Sharma, R.K. (2018) Experimental approaches for defining the role of the Ca2+-modulated ROS-GC system in retinal rods of mouse, in Methods in Molecular Biology 1753: Mouse Retinal Phenotyping – Methods and Protocols, ed, N Tanimoto. Humana Press, New York, pp. 129-158.
Isayama, T. and Makino, C.L. (2012) Pigment mixtures and other determinants of spectral sensitivity of vertebrate retinal photoreceptors, in Photoreceptors: Physiology, Types and Abnormalities, eds. E. Akutagawa and K Ozaki. Nova Science Publishers, pp. 1-31.
Lab Members:

Md Uddin Shahabe Talukder, Ph.D.

Brianna Kim
Past Lab Members:
Rajan Adhikari, Ph.D.
Anaid Perez
Polina Geva, Ph.D.
Amanda Kossoff
Paris Chakravarty
Links:
Contact Us
Department of Pharmacology, Physiology & Biophysics
Chobanian & Avedisian School of Medicine
700 Albany Street W402B
Boston, MA 02118-2526
Phone: (617) 358-8470
e-mail: cmakino@bu.edu