The Hamilton Lab
Research
Membrane biophysics and physiology: fatty acid transport and metabolism.
Magnetic resonance imaging (MRI) of atherosclerotic plaques and fat depots.
Systemic inflammation linking cardiovascular disease, oral inflammation (gum disease), and liver disease: mechanisms and therapies.
Research in our group is aimed at providing fundamental information relating to and interlinking cardiovascular disease, diabetes, obesity, and diseases related to fatty acid metabolism. An overall goal to is to develop novel approaches to biomedical issues by integrating physical-chemical and physiological/biochemical approaches. In our newer research this is achieved by assembling multi-disciplinary teams to translate basic research into clinical applications. We use physical and instrumental methods (including solution state 13C NMR spectroscopy, multidimensional NMR, MR imaging, and fluorescence) that are tailored to the specific questions we are addressing. These techniques are complemented with molecular modeling, molecular biology (measurements of blood and tissue biomarkers), histology, and other cell biology methods.
A. Transport and metabolism of fatty acids
The overall aim of research relating to obesity and diabetes is to describe structural and dynamic aspects of fatty acid binding and transport in plasma, in cell membranes, and in the cytosol with state-of-the-art methods in both structural and cell biology. We study fatty acid binding and transport in the plasma by albumin, their transport across the plasma membrane and their binding to intracellular fatty acid binding proteins (FABP), and the mechanistic links between membrane binding/transport and metabolism.
Specific aims and past research of the fatty acid transport project are illustrated below.
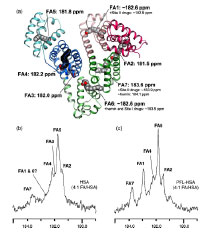
(i) Elucidating molecular details of fatty acid interactions with albumin, primarily by 13C NMR spectroscopy of complexes with 13C-labeled fatty acids and molecular modeling.
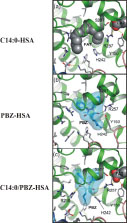
Recently we extended our studies of binding of fatty acids by NMR to an integrated study with NMR, x-ray crystallography, and molecular biology. Illustrated below are results with this integrative approach that have correlated the major binding sites in the crystal structure with NMR peaks from the fatty acid (carboxyl carbon). It is now possible to determine the effect of drugs on fatty acid binding in a site-specific manner, and to locate binding sites of other natural ligands and new drugs by NMR spectroscopy. Current studies are probing the binding sites of steroid hormones by displacement of C-13 fatty acids from their known binding sites.
Extensive publications illustrate the novel design and approaches, and physiological significance including, relevance to disease such as the neurological x-linked disease Adrenoleukodystrophy.
(ii) Determining the solution NMR structure of intracellular fatty acid (FABP) and lipid binding proteins with and without ligands and implications for intracellular metabolism.
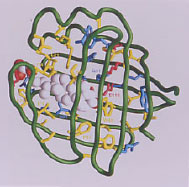

The 3-dimensional structure of the FABP family member ileal lipid binding protein (ILBP) with bound ligand (glycocholate) is illustrated above (left panel). ILBP is much smaller and has a low helical content compared to serum albumin for which NMR solution structure has not been feasible. Our group and others have solved the solution structure for several FABP family members, revealing the same general structural motif as found by x-ray crystallography for several FABP. We have published the complete structure of another FABP, the human intestinal FABP (right panel: cover figure), and we have also determined the structure of a mutation of intestinal FABP associated with diabetes.
(iii) Monitoring movement of natural fatty acids across membranes and desorption from membranes in real time by multiple fluorescence methods.
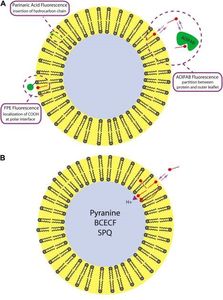
Transport of fatty acids through membranes is a highly active area of current research in cell biology. The figure of a phospholipid bilayer vesicle illustrates some of the methods that are being used and developed in our lab to monitor the movement of fatty acids: a fluorescent-labeled protein ADIFAB in the external buffer and a fluorescent-labeled phospholipid (FPE) to detect fatty acid binding to the outer leaflet of the membrane.
The bottom left panel illustrates novel methods for monitoring of the transmembrane diffuse (flip-flop) of fatty acids using entrapped pH-sensitive dyes. We hypothesize that fatty acid diffusion will result in a pH change inside vesicles (or cells) after addition of external fatty acids.
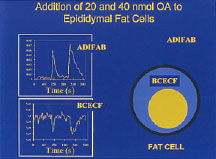
Similar approaches can be applied to live cells to elucidate pathways of entry of fatty acids into cells, as illustrated by the data shown for fat cells with the two probes ADIFAB and the pH dye BCECF (right panel). The increase in ADIFAB fluorescence detects binding of fatty acids to the outer leaflet of the plasma membrane, and the decrease in BCECF fluorescence the inward diffusion across the lipid bilayer to the cytosol,
In our new research, fatty acid-induced pH changes in cells are being examined by whole cell fluorescence and video imaging fluorescence in single cells under conditions modeling diabetes and obesity.
B. In vivo Magnetic resonance imaging (MRI) of atherosclerotic plaques and fat depots
Atherosclerosis is the underlying cause of most acute coronary syndromes (ACS) such as myocardial infarction and unstable angina, which are major causes of mortality in the Western world. Histological studies have demonstrated that ACS are triggered by disruption of vulnerable atherosclerotic plaques which results in luminal thrombosis, but this methodology either requires biopsy or post-mortem analysis.
A major effort in our research has been to develop and apply new NMR and MRI methods for the characterization of lipids in atherosclerotic plaques in vivo before “the horse gets out of the barn,” i.e. an event occurs. The goals include characterizing lipid phases in specific types of plaques, correlating lipid phase and structures to plaque vulnerability to rupture, and providing information for rigorous interpretation of MR images of plaques.
Our research group has developed and optimized MRI for prediction of unstable (high risk plaques) that can cause acute events of atherosclerosis, plaque rupture and thrombosis (atherothrombosis). This project area combines in vivo MRI of live rabbits obtained with the BU Center for Biomedical Imaging (CBI) 3T clinical scanner and ex vivo high resolution images obtained at 11.7T in the High Field Imaging Core Facility.
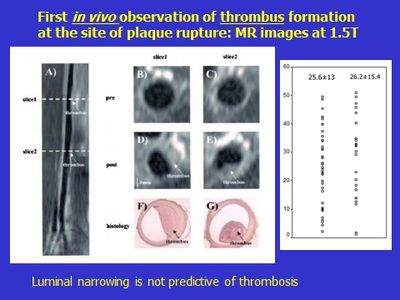
In collaboration with Beth Israel, our group reported the first plaque rupture in a live animal. The images shown are from the same rabbit aorta before (panels A, B, C) and after (D, E) inducing plaque rupture. The axial views illustrate that a new image feature appears after pharmacological inducement (triggering) of thrombosis attached to the lumen. The thrombosis was validated by ex vivo histology (panels F and G). A remarkable finding was that luminal narrowing was not predictive of thrombosis and often occurred in plaques with good luminal flow, as in the example illustrated, and which is recognized a characteristic of human plaques.
The rabbit model of atherothrombosis has been optimized in our lab in recent years. It is now recognized as a well-validated model of human disease. The 3-month protocol accelerates the development of atherosclerosis and produces 6 of the 8 stages of human plaques, unlike other animal models.
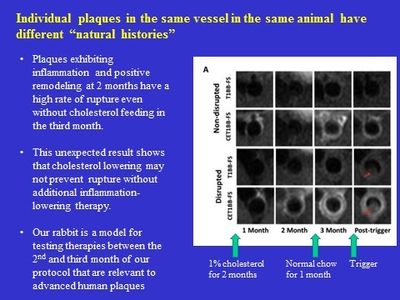
Recently we expanded our imaging studies from images obtained only at the end of the 3 month protocol, as in the above figure, to monthly imaging in vivo at 3T to monitor the progression of individual aortic plaques in the same rabbit (serial imaging).
This produced unique and valuable new insights into plaque development from an earliest stage to advanced highly inflammatory stages. Major findings were that despite returning the rabbit to normal chow at 2 months to achieve dramatic cholesterol lowering, plaques that were highly inflamed at 2 months continued on a pathway of increasing inflammation and risk for rupture.
C. Systemic inflammation linking cardiovascular disease, oral inflammation (gum disease), and liver disease: mechanisms and therapies
With our new discoveries from serial MRI, we have expanded our studies of therapies by natural molecules derived from omega 3 fatty acids and other polyunsaturated fatty acids. These “specialized pro-resolving molecules (SPRM)”, including resolvins and lipoxins, maintain a balance between inflammation and inflammation resolution to achieve healthy homeostasis.
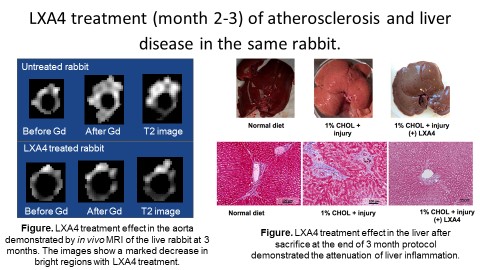
New collaborative studies with the Forsyth Institute have shown that localized oral inflammation can promote and increase inflammation in atherosclerotic plaques. Moreover, localized sites of high inflammation can contribute to chronic systemic inflammation, which can promote disease in other organs, such as the liver (figure below). Our discovery led to testing the therapeutic effects of w-3 fatty acid by stimulation of the resolution of inflammation by SPRM. With oral application of SPRM in the third month of the rabbit protocol as shown in the figure below, we have found beneficial effects in halting and lowering the progression of inflammation in plaques and reversal of liver inflammation and fibrosis. Intracellular liver fat can be quantified by vivo MRS. We are developing protocols for detection of liver fibrosis in our cholesterol fed atherosclerotic rabbits.
Ex vivo Magnetic resonance imaging (MRI) of atherosclerotic plaques and fat depots.
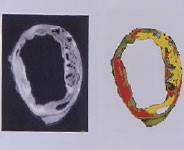

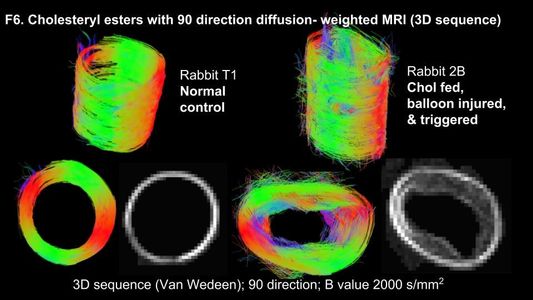
After an atherosclerotic vessel is removed from the body, it can be imaged ex vivo at very high resolution in our core 11.7T MRI. Furthermore, with ex vivo imaging, various pulse sequences can be utilized to highlight different components and fine details of the atherosclerotic plaques, without the time constraints of in vivo imaging. Ex vivo MRI has been a fundamental part of our imaging research and our lab has published several novel application to atherosclerotic plaques.
An example of a complex, advanced plaque in a rabbit artery (with pseudo coloring of the image) is shown in the top left panel. The two images show the different compositional and morphological components as levels of intensity in the conventional grayscale images and a pseudo-colored rendering of the same image section (slice).
With recent advances in imaging technology and programming, technology, we are now able to observe the fine structure of the vessel wall, which is not visible in the conventional images shown above.
JLR cover: A novel application is the use of image-guided NMR spectroscopy and MRS to detect the chemical signature of lipids within a small volume (1cc) in a plaque. Thus, as shown in the cover figure of JLR, we applied this combination method to a human carotid plaque (ex vivo) to show that the regions predicted to be lipid-rich (red) contained the major liquid lipid in plaques, cholesteryl ester.
The major diseases that our lab has studied for years – atherosclerosis, obesity, and type 2 diabetes – are linked by systemic inflammation, which does not cause the initiation of the diseases but promotes disease progression. Meticulous matching of histology slices with high-resolution MRI slices is shedding insights into mechanisms and therapies for inflammatory pathology. We are increasing our focus on blood and tissue biomarkers that identify and quantify disease progression and therapy in our rabbit model of human disease, which now includes Alzheimer’s.
Recent literature reviews of our work
Magnus Back and Göran K. Hansson The FASEB Journal Perspective 2019 Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation.
“These studies showing reduced experimental atherosclerosis after RvE1 administration provide a mechanistic link for w-3 fatty acids to direct anti-atherosclerotic effects by means of stimulation of the resolution of inflammation through the EPA metabolism into RvE1 and downstream signaling transduced through the receptor ERV1/ChemR23.”
Hamilton, J. A., Hasturk, H., Kantarci, A., Serhan, C. N., and Van Dyke, T. (2017) Atherosclerosis, periodontal disease, and treatment with resolvins. Curr. Atheroscler. Rep. 19.
Hansson was elected member of the Royal Swedish Academy of Sciences and served as its secretary general since 2015. Hansson is also a member of the Nobel Assembly at the Karolinska Institute. He has served on the Nobel Committee for Physiology or Medicine (for the Nobel Prize in Physiology or Medicine) for 17 years and was its secretary until 2015. He is Vice Chair of the board of directors of the Nobel Foundation.
Gabrielle Fredman Frontiers in Pharmacology March 2019 Can Inflammation-Resolution Provide Clues to Treat Patients According to Their Plaque Phenotype?
“The first study that demonstrated a therapeutic role for SPMs in atherosclerosis was by Hasturk et al. (2015). This study uncovered that RvE1, when treated orally and topically (i.e., to the gums) thwarted atherogenesis in a rabbit model of atherosclerosis. The oral-topical application suggests a new therapeutic administration strategy for SPMs and is plausible for long-term treatment.”
Hasturk, H., Abdallah, R., Kantarci, A., Nguyen, D., Giordano, N., Hamilton, J., et al. (2015). Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arterioscler. Thromb. Vasc. Biol. 35, 1123–1133.
Links:
Faculty Profile
ResearchGate
Pubmed
Contact Us
James A. Hamilton
Department of Pharmacology, Physiology & Biophysics
Chobanian & Avedisian School of Medicine
700 Albany Street, W302
Boston MA 02118-2526
Phone:(617) 358-8475
e-mail: jhamilt@bu.edu