
Christopher V. Gabel, Ph.D.
Associate Professor of Pharmacology, Physiology & Biophysics
Research
Neurophotonics in Simple Systems
Put simply, the Gabel Lab employs light to study neuroscience in worms. C. elegans, a microscopic nematode, or roundworm, exhibits characteristics that make it ideal for photonic interrogation of the nervous system. The microscopic animal is completely transparent, possesses the simplest of nervous systems and is genetically tractable with a vast library of well-defined mutants and straightforward expression of genetically encoded fluorophores in select neuron types. The C. elegans nervous system consists of 302 stereotyped neurons whose anatomy and connectivity is completely mapped. To this system the Gabel lab brings expertise in high-resolution laser ablation and fluorescence microscopy to study neuronal damage, regeneration and function.
Laser surgery and regeneration of single neurons
Employing a pulsed infrared laser, the Gabel lab performs subcellular ablations within bulk biological tissue. The super-high intensity of a tightly focused femto-second pulsed laser beam results in multi-photon absorption and vaporization of the tissue within the focal volume. At the same time, the low total energy of each light pulse minimizes collateral damaged to the surrounding tissue and minimal linear absorption of the infrared light enables deep beam penetration. Applied to C. elegans this technique enables selective severing of individual nerve fibers within intact adult animals.

Neurological damage presents a major medical challenge, as severed neurons within the mammalian central nervous system typically do not regenerate resulting in permanent and debilitating paralysis. Laser surgery in C. elegans allows in vivo study of neuronal damage and regeneration in a simple highly tractable and reproducible system. The animal’s simple, completely mapped, nervous system allows identification, targeting and severing of a particular neuron at a specific location. Subsequent time-lapse imaging quantifies the degree of regeneration that can be compared across genetic backgrounds, pharmacological treatments and experimental conditions. Armed with these tools the Gabel lab has performed a series of studies decoding the cell intrinsic mechanisms of neuronal damage, recovery and regeneration. Their studies have defined the role of apoptotic caspases (nominally known for mediating programed cell death during development) in regeneration (Pinan-Lucarre et al. PLoS Biology 2012). As a post-doc in the lab, Dr. Samual Chung, (currently an Assistant Professor of Bioengineering at Northeastern University) spear headed a study defining the effects of neuronal activity on regeneration (Chung et al. PNAS 2016). More recently, in a project initiated by Dr. Dan Taub, a former graduate student in the lab, the group has focused on the fundamental importance of cellular metabolism in dictating a neurons regenerative capacity. Notably, they have discovered post-translational protein modifications that can alter cellular metabolism to greatly enhance neuronal regeneration (Taub et al. Cell Reports 2018).
Neuronal imaging from single cell physiology to whole brain function
Calcium is a key second messenger modulating a wide range of cellular processes in neurons from synaptic release, to gene expression, to axon guidance. Genetically encoded fluorophores allow in vivo imaging of calcium dynamics on the cellular and subcellular scale. The Gabel lab has adapted these techniques to measure subcellular calcium dynamics within a damaged and regenerating neuron in C. elegans. By laser severing and imaging a neuron expressing calcium sensitive G-CaMP, they have identified sustained sub-cellular calcium signals and the cellular components modulating them which are critical to efficient regeneration. Moreover employing optogenetic light activated channels the Gabel lab was able to manipulate these signals enhancing the neurons regenerative capacity (Sun et al. J. Neuroscience 2014). Thus using modern neurophotonics the Gabel lab can effectively damage an individual neuron, measure its physiological response and modulate that response to improve neuronal recovery.
Calcium fluorophores are also employed to measure neuronal activity, with indicators reporting the elevation of cellular calcium resulting from neuronal depolarization. The Gabel lab has recently developed techniques to measure neuronal activity in C. elegans within pathological context such as physical damage and age related decline. In collaboration with Professor Chris Connor (Dept. of Anesthesiology, Brigham and Women’s Hospital, Harvard University), the lab recently acquired a diSPIM light sheet microscope capable of fast volumetric imaging of the entire head of C. elegans. The small size of its nervous system and cell specific expression of calcium indicators allows imaging of individual neurons, specific well-defined circuits controlling behavior or the activity across the majority of the nervous system with single cell resolution.
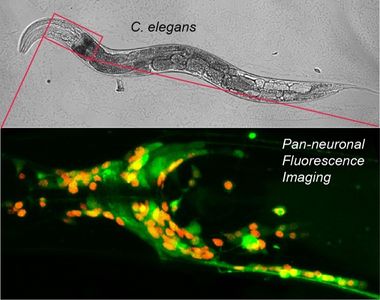
Work with Dr. Connor is focused on the effects of volatile anesthetics on neuronal function. Despite their prevalence in modern medicine the mode of action of volatile anesthetics on the nervous system is largely unknown. In addition, they present poorly defined risks to the development of the very young and to sustained cognition in the very old. C. elegans is an established model system in the search for anesthetic receptors and displays behavioral phases of anesthetization similar to humans. Functional neuronal imaging in C. elegans presents an opportunity to understand how volatile anesthetics disrupt neuronal function in a controlled and reversible manor. In a recent study performed by Mehraj Awal, a graduate student in Physiology and Biophysics and NRT fellow, the group showed that neurons within a highly ordered sub-circuit of C. elegans controlling behavior becomes disorganized and hyperactive with the onset of anesthesia (Awal et al. Anesthesiology 2018). More recently Greg Wirak, a GPN student and NRT fellow in the lab, has discovered that exposure to volatile anesthetics during development altars the neuronal dynamics of key interneurons in the adult animal. These findings open a new window into the potential health risks of anesthetics in the very young. Moving forward the Gabel lab is working to expand these techniques to pan-neuronal imaging across the majority of neurons in the animal’s head, in order to understand the effects of volatile anesthetics on a systems level and how this correlates with measurements made on human patients (EEGs).