The Isoaspartome
Deamidation of asparagine, Asn (and to a lesser extent glutamine, Gln) residues is the most common post-translation modification (PTM), occurring in many proteins as a non-enzymatic, spontaneous reaction. Deamidation plays an important role in aging, autoimmune disorders (e.g Celiac Disease), neurodegeneration (e.g. Alzheimer’s Disease), and other diseases; it can also influence the stability and potency of protein-based drugs. Under physiological conditions, Asn deamidation proceeds via a succinimide intermediate, which hydrolyzes to form a mixture of aspartate (Asp) and isoaspartate (isoAsp) residues.
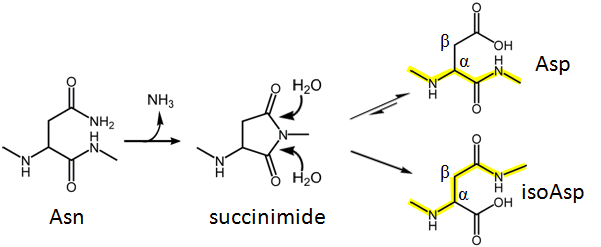
Despite its ubiquity and importance in life science and in pharmaceutical industry, deamidation is underappreciated and understudied. This is in part due to the difficulty in differentiating the deamidation products, as Asp and isoAsp residues have the same mass and similar physiochemical properties. It was recently shown that electron capture dissociation (ECD) can cleave the Cα-Cβ bond in these residues resulting in diagnostic marker peaks in the MS/MS spectra. For aspartic acid, it produces a M-60 peak, for isoaspartic acid, it produces c+57, z-57 peaks.
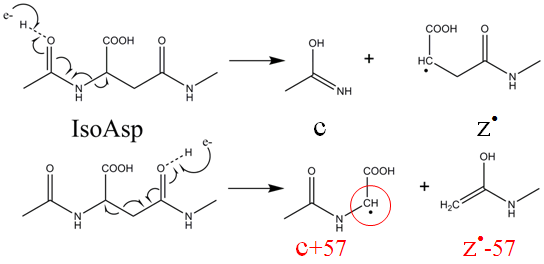
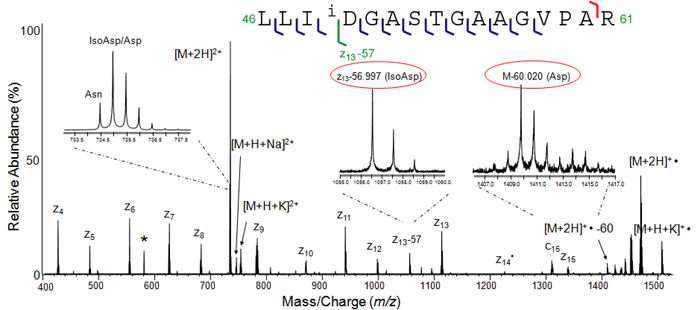
To demonstrate this, a light chain peptide (46-61) from a patient with Primary Systemic Amyloidosis was subjected to ECD analysis, which generated isoAsp diagnostic z13-57 and Asp diagnostic M-60 ions, indicating the Asn49 residue has deamidated to form a mixture of Asp and isoAsp residues.
Recent progresses of this work include the development of other electron activated dissociation methods, such as electron impact/ionization dissociation (EID) and electron transfer dissociation (ETD) for isoAsp analysis, as well as for the related studies of glutamine deamidation (γ-Glu identification). The potential of this method in the relative quantitation of isoAsp content, in the analysis of N-terminal isoAsp residues, and in analyzing HPLC-separated deamidation mixtures has been demonstrated. IsoAsp analysis employing top-down, middle-down, and MS3 approaches is now possible. The ECD method has been successfully applied to monitor isoAsp formation in A-beta peptides, in proteins from patient samples, and in stressed protein-based drugs (e.g. Anthrax Vaccine). However, ECD does not generate diagnostic ions for characterization of β-amino acid residues other than isoAsp and β-phenylalanine due to the lack of radical stabilization. We have recently showed that MALDI in-source decay (ISD) with a hydrogen-accepting matrix can be broadly applied for β-peptide characterization by producing site-specific a-14 ions at β-linkage sites.
The following papers develop this work in detail:
- Yu, X.; Sargaeva, N. P.; Thompson, C. J.; Costello, C. E.; Lin, C. In-Source Decay Characterization of Isoaspartate and β-Peptides Int. J. Mass Spectrom. 2015, 390, 101-109.
- Li, X.; Yu, X.; Costello, C. E.; O’Connor, P. B. Top-Down Study of β2-Microglobulin Deamidation Anal. Chem. 2012, 84 (14), 6150-6157.
- Sargaeva, N. P.; Lin, C.; O’Connor, P. B. Differentiating N-Terminal Aspartic and Isoaspartic Acid Residues in Peptides Anal. Chem. 2011, 83, 6675. Link
- Sargaeva, N. P.; Lin, C.; O’Connor, P. B. Unusual Fragmentation of β-Linked Peptides by ExD Tandem Mass Spectrometry J. Am. Soc. Mass Spectrom. 2011, 22, 480. Link
- Sargaeva, N. P.; Goloborodko, A. A.; O’connor, P. B.; Moskovets, E.; Gorshkov, M. V. Sequence-Specific Predictive Chromatography to Assist Mass Spectrometric Analysis of Asparagine Deamidation and Aspartate Isomerization in Peptides Electrophoresis, 2011, 32, 1962. Link
- Li, X.; Lin, C.; O’Connor, P. B. Glutamine Deamidation: Differentiation of Glutamic Acid and γ-Glutamic Acid in Peptides by Electron Capture Dissociation Anal. Chem. 2010, 82, 3606. Link
- Sargaeva, N. P.; Lin, C.; O’Connor, P. B. Identification of Aspartic and Isoaspartic Acid Residues in Amyloid-β Peptides, Including Aβ 1-42, Using Electron-Ion Reactions Anal. Chem. 2009, 81, 9778. Link
- Li, X.; Cournoyer, J. J.; Lin, C.; O’Connor, P. B. Use of 18O Labels to Monitor Deamidation during Protein and Peptide Sample Processing J. Am. Soc. Mass Spectrom. 2008, 19, 855. Link
- Cournoyer, J. J.; Lin, C.; Bowman, M. J.; O’Connor, P. B. Quantitating the Relative Abundance of Isoaspartyl Residues in Deamidated Proteins by Electron Capture Dissociation J. Am. Soc. Mass Spectrom. 2007, 18, 48. Link.
- O’Connor, P. B.; Cournoyer, J. J.; Pitteri, S. J.; Chrisman, P. A.; Mcluckey, S. A. Differentiation of Aspartic and Isoaspartic acids Using Electron Transfer Dissociation J. Am. Soc. Mass Spectrom. 2006, 15-19 Link.
- Cournoyer, J. J.; Lin, C.; O’Connor, P. B. Detecting Deamidation Products in Proteins by Electron Capture Dissociation Anal. Chem. 2006, 78, 1264. Link.
- Cournoyer, J. J.; Pittman, J. L.; Ivleva, V. B.; Fallows, E.; Waskell, L.; Costello, C. E.; O’Connor, P. B. Deamidation: Differentiation of Aspartyl from Isoaspartyl Products in Peptides by Electron Capture Dissociation Protein Science 2005, 14, 452-463 Link.