Acylproteome
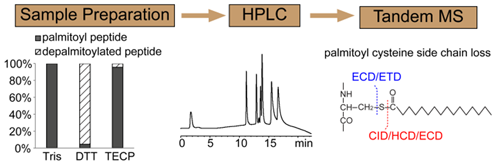
Protein S-palmitoylation results from the covalent attachment of a palmitate to the cysteine sulfhydryl group via a labile thioester linkage. S-palmitoylation is reversible and dynamic, and plays an important regulatory role in protein trafficking, membrane localization, and cellular signaling. S-palmitoylation is traditionally detected by radioisotope labeling, but the procedure is toxic, time consuming, and generally cannot specify the palmitoylation sites. An alternative approach utilizing metabolic labeling and click chemistry has shown great promise in large-scale profiling of protein palmitoylation using cell lines, but cannot be easily extended to study palmitoylation in large organisms. Indirect detection methods utilizing the acyl-biotinyl exchange chemistry have also been applied to study S-palmitoylation, but false discovery of palmitoylation may arise due to incomplete blockage of free cysteine thiol(s) and/or the presence of other types of acylation. Meanwhile, direct detection and quantification of S-palmitoylation by mass spectrometry is challenging because of the tendency of palmitoyl loss during sample preparation and tandem MS analysis.
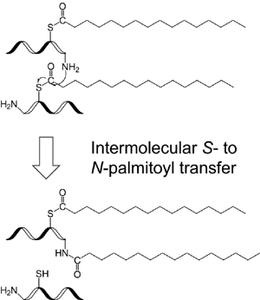
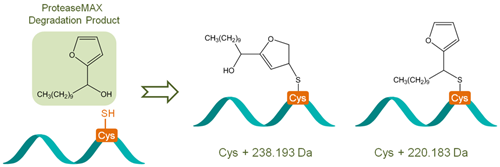
We have recently developed a sample preparation protocol that preserves S-palmitoylation for on-line LC-MS/MS analysis and a derivatization strategy that allows relative quantification of protein palmitoylation. We showed that our approach has sensitivity comparable to the traditional radioactive labeling method, and does not suffer from some of the limitations that are inherent to metabolic labeling approaches. We discovered that during proteomic sample preparation, the S-palmitoyl group may migrate to an amino group at the protein N-terminus or on the lysine side chain, potentially leading to false discovery of N-palmitoylation. We showed that addition of detergent stabilizes S-palmitoylation, thus preventing inter-molecular S– to N-palmitoyl transfer and based-catalyzed palmitoyl loss. However, the choice of detergent is crucial as the use of certain MS-compatible acid-labile surfactant can lead to artifactual modifications of the cysteine thiol that could be misinterpreted as palmitoylation or other lipid modifications.
Our current effort is focused on the development of palmitoyl peptide enrichment methods. We will also explore the potential of direct palmitoyl analysis by MS in biological systems.
The following papers describe this work in detail:
- Ji, Y.; Bachschmid, M. M.; Costello, C. E.; Lin, C. S– to N-Palmitoyl Transfer during Proteomic Sample Preparation J. Am. Soc. Mass Spectrom. 2016, 27(4), 677-685.
- Ji, Y.; Liu, M.; Bachschmid, M. M.; Costello, C. E.; Lin, C. Surfactant-Induced Artifacts during Proteomic Sample Preparation Anal. Chem. 2015, 87 (11), 5500-5504.
- Ji, Y.; Leymarie, N.; Haeussler, D. J.; Bachschmid, M. M.; Costello, C. E.; Lin, C. Direct Detection of S-Palmitoylation by Mass Spectrometry Anal. Chem. 2013, 85 (24), 11952-11959.