Glycan-ExD-Mechanism
Conventional slow-heating fragmentation methods often fail to produce abundant informative cross-ring fragments needed for detailed glycan structural characterization, particularly determination of the branching pattern and linkage types. Recently, several electron-activated dissociation (ExD) techniques have been applied to glycan structural analysis, and have shown great promise. It was further shown that the glycan ExD fragmentation behavior depends on a number of experimental parameters and structural variables. However, these methods remain poorly characterized and their potential underexplored. A theoretical investigation could shed important light on the glycan ExD fragmentation mechanism. A sound understanding of the ExD process is crucial for the development of a high-throughput, high-sensitivity method for routine glycan structural analysis, as well as for successful development of bioinformatics tools for automatic, de novo glycan structural characterization.
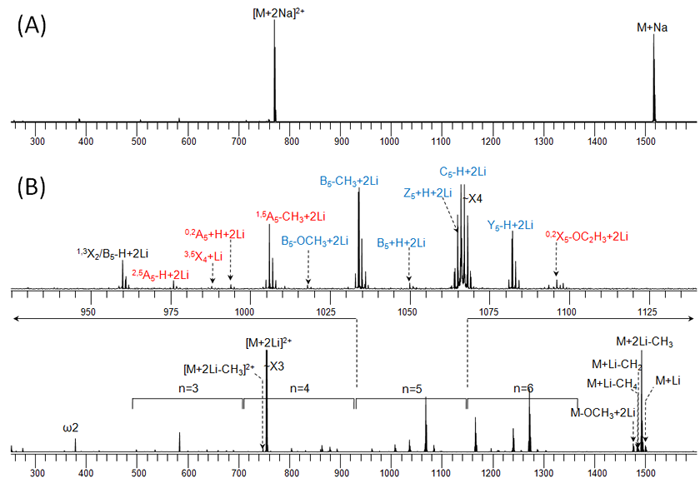
The following figure illustrates the influence of the cationizing agent on the ECD fragmentation behavior of a metal-adducted permethylated model oligosaccharide (maltoheptaose): (A) 1.5 eV ECD spectrum of the sodium adduct; (B) 1.5 eV ECD spectrum of the lithium adduct.
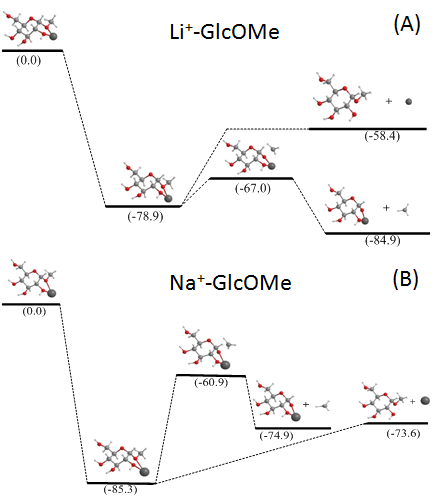
The charge carrier-dependent fragmentation behavior can be understood by theoretical modleing on small model systems: alkali-metal coordinated β-methyl-glucosides (GlcOMe). The potential energy surfaces (PESs) for the metal loss and methyl loss channels were calculated using density functional theory (DFT), and shown in the following figure: (A) lithium adduct; (B) sodium adduct. For the lithium adduct, methyl loss is both kinetically and thermodynamically favored, whereas for the sodium adduct, although the two channels have similar exothermicities, the metal loss is kinetically favored.
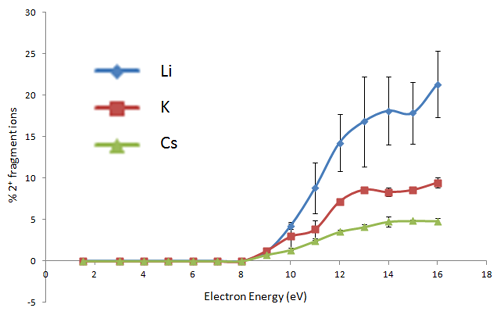
ExD fragmentation behavior of metal-adducted glycans also shows a strong dependence on the electron energy used, with the fragmentation pathway changing from low-energy ECD (~1 eV) to hotECD (hECD) at intermediate energies (~6 eV), electronic excitation dissociation (EED) at high energies (>10 eV). The following figure shows the energy dependence of the relative abundance of doubly-charged fragment ions formed from ExD of a doubly-metal adducted precursor ion (permethylated maltoheptaose). Since doubly-charged fragment ions can only result from the EED process, the onset of EED can be determined to be at ~9 eV, independent of the charge carriers, and close to the first ionization potential of dimethyl ether (~10 eV): the small energy difference likely arises from the small kinetic energy distribution of electrons from the indirectly heated cathode dispensor.
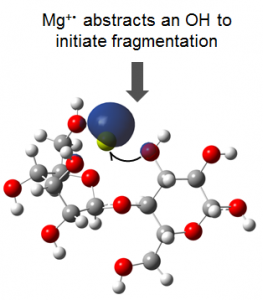
Our theoretical investigation revealed that ECD of metal-adducted glycans is initiated by electron capture at a metal binding site, followed by abstraction of a spatially adjacent hydroxyl group, generating a carbon-centered radical that subsequently undergoes sequential hydrogen migration(s) and/or alpha cleavages to form a variety of product ions. The figure below shows the frontier singly occupied molecular orbital of the charge reduced cellobiose-Mg2+ complex.
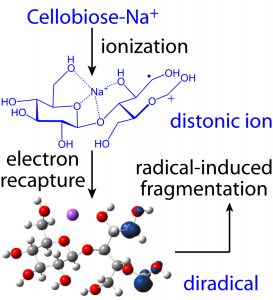
Irradiation of a metal-adducted glycan by higher-energy electrons (> 10 eV) leads to ionization and formation of a distonic ion. The distonic ion then captures a slow electron to generate a di-radical with trivial singlet-triplet splitting. The triplet state di-radical subsequently undergoes radical-induced fragmentation.
Relevant Publications:
- Huang, Y.; Pu, Y.; Yu, X.; Costello, C. E.; Lin, C., Mechanistic Study on Electronic Excitation Dissociation of the Cellobiose-Na+ Complex, J. Am. Soc. Mass Spectrom. 2016, 27, 319-328.
- Huang, Y.; Pu, Y.; Yu, X.; Costello, C. E.; Lin, C., Mechanistic Study on Electron Capture Dissociation of the Oligosaccharide-Mg2+ Complex, J. Am. Soc. Mass Spectrom. 2014, 25, 1451-1460.
- Yu, X.; Huang, Y.; Lin, C.; Costello, C. E., Energy-Dependent Electron Activated Dissociation of Metal-Adducted Permethylated Oligosaccharides, Anal. Chem. 2012, 84 (12), 7487-7494.