Unraveling Alzheimer’s Disease // Part 2 The Search for a Treatment
Clinical trials aimed at attacking the ailment before symptoms occur

Alzheimer’s disease is an epidemic. It attacks the brain’s nerve cells, causing memory loss, behavioral changes, confusion, and deterioration of language skills. It affects more than 5 million Americans 65 and older, and that is expected to increase to 13.8 million by 2050 unless science finds a treatment. Alzheimer’s and other forms of dementia are projected to cost the nation $236 billion this year and the figure could reach $1 trillion by 2050, according to the Alzheimer’s Association.
At Boston University, dozens of researchers are looking for tests that could lead to early diagnosis and interventions to prevent or delay the disease, running clinical trials that may result in treatments and an eventual cure, working to understand genetic risk factors, and studying Alzheimer’s impact on caregivers.
Boston University Alzheimer’s Disease Center, established in 1996, is one of 31 such centers nationwide funded by the National Institutes of Health and dedicated to conducting research into the disease, enhancing clinical care, and providing education.
In this special report, BU Today examines the work of five BU researchers.
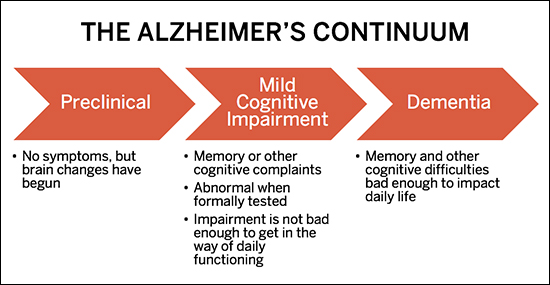
The challenge of developing a treatment for Alzheimer’s, says BU’s Robert Stern, is that once symptoms develop, existing drug interventions deliver too little too late. For those fated to develop the disease, changes in the brain happen as long as 15 to 25 years before any outward signs of impairment, such as memory loss or inability to perform cognitive tasks. Alzheimer’s is not an abrupt onset condition, but an insidious continuum, from no symptoms (preclinical phase) to mild memory loss and other cognitive difficulties (mild cognitive impairment, or MCI phase) to more pronounced memory and cognitive impairment, severe enough to impact independent functioning (dementia phase). By the time Alzheimer’s causes dementia, the brain is damaged beyond repair.
Autopsies have long confirmed the ways Alzheimer’s ravages the brain. The cerebral cortex, the brain region involved in thinking, planning, and remembering, gradually shrivels up. In the hippocampus, which plays an important role in the formation of new memories, shrinkage is severe. The fluid-filled spaces in the brain grow larger, and abnormal, sticky clusters, or plaques, of beta-amyloid, a toxic protein, aggregate between neurons. This makes it impossible for them to communicate in what in the healthy brain is a vast, intricate circuit board of cells that transmit the nerve impulses that control everything we do, think, and feel, voluntary and involuntary. Most researchers currently believe that the beta-amyloid plaques begin a destructive cascade of events in the brain, including the buildup of another bad protein, called tau, inside the brain cells, ultimately leading to the death of those cells, inflammatory responses, progressive atrophy of the brain, worsening cognitive functioning, and ultimately, death.
“There hasn’t been a new Food and Drug Administration–approved Alzheimer’s treatment for about 12 years,” says Stern. “There’s this gigantic need to find a meaningful treatment.”
It’s a grim picture. But Stern, a School of Medicine professor of neurology, neurosurgery, and anatomy and neurobiology, and director of Alzheimer’s Disease Center (BU ADC) Clinical Core, is hopeful that Alzheimer’s will become a treatable disease. As one of his many responsibilities, he oversees a series of clinical trials, funded by both the National Institutes of Health (NIH) and industry, that focus on the effectiveness of experimental drugs to treat Alzheimer’s at all stages of the disease, even before its ensuing downhill slide ending in full-blown dementia. What fuels the optimism of Stern, his team, and others around the world collaborating in these clinical trials is that there are now ways to identify markers in the brain that have been shown to be strong indicators that someone has Alzheimer’s and will develop worsening symptoms. This, says Stern, means that people whose brain scans indicate a high risk of the disease could be treated before they are symptomatic. By targeting those destructive beta-amyloid plaques, these experimental compounds could prove effective in minimizing or arresting the disease’s assault on the brain.
Current treatments for Alzheimer’s-induced dementia are no match for the brain’s deterioration. Stern and his team’s current clinical trials—reflecting intervention at different points along the Alzheimer’s continuum—are comparing experimental drugs to placebo, measuring the effectiveness of potential drug therapies aimed at removing beta-amyloid from the brain or protecting the brain cells from the toxic effects of the protein. All of these studies involve carefully screened volunteers recruited through the BU ADC. All of the clinical studies dovetail with the center’s flagship HOPE (Heath Outreach for the Elderly) study, which is a registry of research participants to help researchers who are studying normal aging and Alzheimer’s disease.
Like Sisyphus, fighting an uphill battle
Stern is emphatic that clinical trials must be stepped up. With 330 baby boomers turning 60 every hour, a public health crisis looms.
“There hasn’t been a new Food and Drug Administration–approved Alzheimer’s treatment for about 12 years, and the problem is only going to grow exponentially over the next decade,” he says. “There’s this gigantic need to find a meaningful treatment.” And what he calls a preclinical treatment would offer the greatest promise.
Anyone who has a loved one with Alzheimer’s is most likely familiar with the drug Aricept (generic name: donepezil). But it does not slow the progression of, or cure, the disease; it provides only small improvements in small numbers of patients, says Stern. That drug and a handful of others “are still being used and prescribed, as they should be. But none do anything to actually modify the disease course; they weren’t designed to do anything to slow down the disease, which is currently the only disease among the top 10 causes of death in this country that can’t be cured, prevented, or slowed down.” Like Sisyphus, he says, drugs like Aricept are fighting an uphill battle as more and more cells are being destroyed, until the drugs have no effect at all. In contrast, the new drugs being examined in current clinical trials aim to slow the course of the disease by intervening in some aspect along the course of its destructive descent.
Alzheimer’s Continuum chart showing the stages of the disease
The promise of early intervention: Alzheimer’s-related changes in the brain can happen as much as 25 years before any outward signs of impairment. At the BU Alzheimer’s Disease Center, Robert Stern and his team of clinical researchers are testing new drugs that may offer hope for early intervention.
One factor fueling Stern’s optimism is the usefulness, and broadened use (the cost is still prohibitive for most) of positron emission tomography (PET) scans to identify bad amyloid protein deposits in asymptomatic people. What if drugs could be administered early on, in the first stage of the disease, when there’s a positive brain scan but no outward evidence of impairment? A type of nuclear medicine imaging test, PET scans have been around in some form since the 1960s. The scan uses a radioactive substance called a tracer to look for disease in the body. But now there are three FDA-approved PET scans specifically created to detect amyloid plaques in the brain. “We can put research participants in the PET scanner, and we can see where it lights up,” Stern says, pointing to a healthy brain scan and one with significant bad amyloid buildup, which in the computer screen’s color-enhanced images appear to be forging tributaries deep into the brain tissue.
The BU ADC studies are part of several major clinical studies in different phases, funded by the NIH and several pharmaceutical companies, of drugs that might work, alone or in combination, to spare people the long, costly decline of Alzheimer’s-related dementia. One, called the A4 Study, is examining the effects of the anti-amyloid drug Solanezumab in individuals in the preclinical phase of the disease—that is, without memory or cognitive impairment, but showing evidence of elevated beta-amyloid on a PET scan. Another, Expedition 3, is examining that same anti-amyloid drug in participants in the early symptomatic stages of Alzheimer’s disease. The BAN2401 study is looking at the effects of another anti-amyloid compound in patients with MCI and very early Alzheimer’s dementia. And the NOBLE study is assessing the effects of an experimental oral medication on people with mild to moderate Alzheimer’s dementia who are currently on Aricept. Specifically, the NOBLE study is assessing the effectiveness, compared with placebo, of the experimental drug drug T-817MA , which is designed to protect neurons from the toxic insults and may actually sprout new connections between existing neurons, Stern says.
On a soap box
Most of the drugs Stern’s group is testing are anti-amyloid antibody drugs, monoclonal antibodies developed over the last decade specifically for use in treating Alzheimer’s. These are customized antibodies produced by single clones of cells or cell lines. Study participants undergo intravenous infusion of the drug once or twice a month. If the drug reaches the brain, research indicates it attaches to the bad amyloid and “sucks it out of the brain,” says Stern.
It’s unlikely that a single drug will work for everyone, he says. But his goal is to one day be able to personalize treatment so drug administration will be tailored to the point a person is on the Alzheimer’s continuum. “The idea would be to try to select the drugs for the specific time point for that specific patient,” he says.
“In addition to providing much-needed hope, participation in research also makes people feel connected and less alone during what is often a lonely journey with this disease,” says Stern.
The BU ADC tries to recruit at least 10 volunteers for each study. Recruitment coordinator Diane Essis says there has been an enthusiastic response from those willing to be screened. “People who are enrolled in these studies want to be in these studies,” most lasting at least two to three years. For early to moderate Alzheimer’s patients in studies involving the use of a placebo, Essis says, “I like to stress that they’ve progressed several stages into the disease and this is something that may help them and will almost certainly help others, perhaps even family members down the line. And participating in research is a way to be proactive.” Stern says that “in addition to providing much-needed hope, participation in research also makes people feel connected and less alone during what is often a lonely journey with this disease.” And of course, these studies pave the road to FDA approval.
What about potential negative side effects? “Here’s where I get on my soap box,” says Stern. “Alzheimer’s is a fatal disease that robs people of so much—from being who they were, from living life the way they did. It’s a fatal disease that robs people of everything and yet the amount of risk that research participants and regulators are willing to take historically has been minimal, because we’ve only been thinking about it as a part of normal aging or a disease in very old people.” He stresses that Alzheimer’s is not part of normal aging. “It is a bad brain disease that can affect people in their 50s or early 60s, with the disease starting decades before symptoms.”
As for clinical research, there’s a need for what he calls a paradigm shift. In cancer trials, for example, people are “not just expecting, but willing, to have significant side effects,” says Stern, whose first wife passed away from cancer at age 42. “We know that people are willing to do anything for cancer treatment or clinical trials, and yet when it comes to this other fatal disease that robs us of everything, there is less willingness to have any side effects from treatments.” The goal, he stresses, is to find effective treatments with the least side effects, but “there may be a need to accept some minimal side effects from new medications that can truly combat this devastating disease.”
Stern doesn’t foresee an Alzheimer’s cure in the traditional sense. “Right now in our understanding of how the brain works, when you’ve already lost brain tissue, magic medication won’t bring back brain tissue and function—but I’d love being found wrong in the future,” he says. But even without a cure in the traditional sense, “if there are methods that are truly successful in slowing down the disease, or halting its progression, and they can be started very early in the disease course, before symptoms begin, then in some ways we’ve cured it.”
Read the other stories in our “Unraveling Alzheimer’s Disease” series here.
This BU Today story was written by Susan Seligson.