Unraveling Alzheimer’s Disease // Part 1 Diagnosis
Looking for a better way to detect the disease early

Alzheimer’s disease is an epidemic. It attacks the brain’s nerve cells, causing memory loss, behavioral changes, confusion, and deterioration of language skills. It affects more than 5 million Americans 65 and older, and that is expected to increase to 13.8 million by 2050 unless science finds a treatment. Alzheimer’s and other forms of dementia are projected to cost the nation $236 billion this year and the figure could reach $1 trillion by 2050, according to the Alzheimer’s Association.
At Boston University, dozens of researchers are looking for tests that could lead to early diagnosis and interventions to prevent or delay the disease, running clinical trials that may result in treatments and an eventual cure, working to understand genetic risk factors, and studying Alzheimer’s impact on caregivers.
The Boston University Alzheimer’s Disease Center, established in 1996, is one of 31 such centers nationwide funded by the National Institutes of Health and dedicated to conducting research into the disease, enhancing clinical care, and providing education.
In this special report, BU Today examines the work of five BU researchers.
When a patient arrives at the ER with a suspected heart attack, doctors run down a list of symptoms: unremitting chest pain, days of fatigue, indigestion. But cardiologists don’t stop there: they can order blood tests, an EKG, an echocardiogram. With all the information in hand, doctors can make a definitive diagnosis: heart attack, or no.
Physicians trying to diagnose dementia have far fewer tools at their disposal. “Diagnosing Alzheimer’s patients is a challenge,” says Wendy Qiu, a School of Medicine associate professor of psychiatry and pharmacology, and principal investigator in the Laboratory of Molecular Psychiatry in Aging. “Clinically we still depend on symptoms, so that sometimes we can be wrong.” Qiu’s goal: to help create quick, cheap, reliable tests that make early-stage Alzheimer’s as easy to diagnose as a heart attack.
“If you can’t diagnose it, you can’t identify people with the disease and get them into clinical trials, and that’s our best hope for finding a treatment,” says Neil Kowall, a MED professor of neurology and pathology and director of BU’s Alzheimer’s Disease Center, where Qiu is on the faculty. “How can you treat something you can’t even identify?”
One obstacle to easy diagnosis is something in our bodies called the blood-brain barrier, a sheen of tightly bound cells that separates the blood circulating through our body from the cerebrospinal fluid washing around our brain and spine. The blood-brain barrier allows water and some gases through, as well as molecules like glucose that are critical for brain function. But many molecules, including some that may be useful for diagnosing Alzheimer’s, get stuck on the brain side of the barrier, so never appear in a blood test.
“If you can’t diagnose it, you can’t identify people with the disease and get them into clinical trials, and that’s our best hope for finding a treatment,” says Neil Kowall. “How can you treat something you can’t even identify?”
One way around this problem is a lumbar puncture: a clinician slides a needle into a patient’s spinal column and takes a small sample of cerebrospinal fluid (CSF). Scientists then look for two Alzheimer’s biomarkers in the fluid: high levels of phosphorylated tau, a protein that sticks together in tangles, and low levels of amyloid beta-42, a protein fragment that clumps into plaques. (Scientists guess that when amyloid beta-42 deposits in the brain, less of it appears in the CSF.) These tests are fairly accurate, but scientists now use them mostly for research, not diagnosis. That’s because few patients are clamoring for a spinal tap.
“Patients are really reluctant to have a lumbar puncture. It’s painful,” says Qiu. “Especially at the early stage of Alzheimer’s disease, when they have minor memory complaints, they don’t want to have that.”
A PET (positron emission tomography) scan, which can detect beta-amyloid plaques, is painless but expensive: up to $18,000 per scan. “Considering that currently we have more than five million Alzheimer’s patients, and as the population ages, we’re going to have more, we just cannot afford to have these kind of scans,” says Qiu.
What Qiu—and everyone, really—wants is a simple, reliable blood test that will allow primary care doctors to screen for early markers of Alzheimer’s the way they measure cholesterol. But because of the blood-brain barrier problem, Qiu needs a proxy, something she can measure in the blood that reveals pathology in the brain. To find one, she took a closer look at how the body makes those sticky beta-amyloid plaques. Researchers know that the process begins with a larger molecule called amyloid precursor protein, or APP. Through a series of biochemical steps—some known, some not—the body chops APP into beta-amyloid.
“Most people in the field were focusing on how beta-amyloid is produced from APP,” says Qiu, whose work is funded by the National Institutes of Health and the Alzheimer’s Association. “But I was interested in how beta-amyloid is further degraded after it is formed.” That’s because if the beta-amyloid gets chopped into even smaller bits, it dissipates harmlessly, rather than clumping into neuron-choking plaques. After years of detective work, Qiu found that the enzyme that chops up beta-amyloid is the same one that degrades insulin. It’s called, aptly enough, insulin-degrading enzyme.
Scientists have known for a long time that diabetes, which initially increases blood insulin levels before the pancreas falters, amplifies the risk of Alzheimer’s. Now Qiu thought she knew why: higher insulin in the blood uses up more insulin-degrading enzyme, so less is available for chopping beta-amyloid. Then the excess beta-amyloid forms Alzheimer’s plaques. It made sense, but when she tested the idea, it didn’t pan out. She was stumped. The hypothesis had seemed so perfect: what was going on?
Then she had an idea: amylin. Amylin, like insulin, is a hormone produced in the pancreas that regulates appetite and glucose. But amylin has one important difference from its better-known cousin: insulin barely crosses the blood-brain barrier, but amylin crosses with ease. Aha, thought Qiu, it all makes sense: higher amylin in the brain equals higher beta-amyloid, which leads to Alzheimer’s. Amylin was also known to aggregate in the brains of Alzheimer’s patients, forming its own toxic plaques; now it seemed like an all-around troublemaker. “We thought higher amylin must be bad for Alzheimer’s disease,” Qiu says. “We just wanted to prove it.”
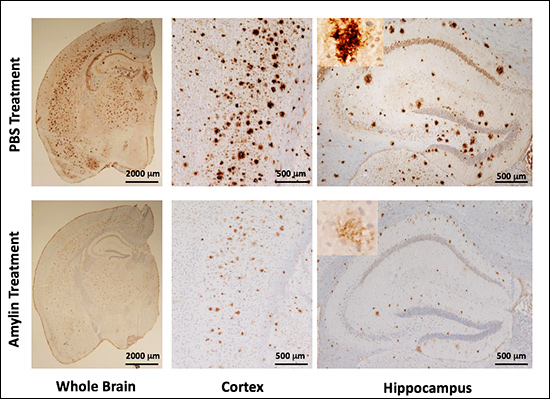
So Qiu and colleague Haihao Zhu, a research scientist in the Qiu lab, tested the hypothesis, using special mice bred to develop Alzheimer’s. The scientists gave half the mice injections of amylin every day for 10 weeks, the other half—the control group—got saline injections. After a few months, the control mice developed Alzheimer’s as expected, their brains smattered with amyloid plaques. But the amylin mice, the ones Qiu expected to develop much more severe Alzheimer’s, had far fewer plaques.
“It completely surprised us. It’s the opposite of what we thought,” she says. The results, published in Molecular Psychiatry in 2014, showed that amylin not only reduced amyloid plaques, it also made the Alzheimer’s mice smarter, improving their learning and memory. Somehow, injections of amylin moved beta-amyloid out of the brain and into the bloodstream, where it does less harm and can be detected. Work that at first seemed a dead end now offers hope for a diagnostic blood test.
“It’s only the unexpected things that push you in the right direction. That’s where progress comes,” says Kowall. “When a scientist is smart, careful, and keeps her eyes open, that’s when the breakthroughs happen.”
“I think the goal of medicine is to reduce the pain of mankind. I strongly believe that,” says Qiu. “We can do almost nothing for Alzheimer’s patients right now. I want to see what we can do for them.”
Qiu is now preparing a pilot study to test amylin in humans. She’s using a synthetic version, called pramlintide, that scientists developed a decade ago to treat diabetes. The synthetic version, which is approved by the Food and Drug Administration, reduces appetite and helps manage glucose, but also has a slightly altered structure that prevents it from aggregating.
“It makes everything easier that it’s already FDA-approved and it’s already in the clinic,” says Qiu. She adds, however, that because the patent on synthetic amylin will expire in 2019, pharmaceutical companies have little interest in supporting research such as hers, which studies the effects of a repurposed drug.
But Kowall says that the benefits of using a drug that’s already on the market may outweigh the drawbacks. “The fact that it’s FDA-approved gives it a big leg up,” he says, and Qiu’s progress with synthetic amylin may lead pharmaceutical companies to pursue similar lines of research. “Pramlintide may just be the first generation,” he says. “It may lead to other drugs that work differently, or better.”
Ultimately, Qiu hopes that her work with amylin will lead not only to a simple blood screen, but also to a treatment. Although that’s still years away, she’s cautiously optimistic. “I think the goal of medicine is to reduce the pain of mankind. I strongly believe that,” she says. “We can do almost nothing for Alzheimer’s patients right now. I want to see what we can do for them.”
Read the other stories in our “Unraveling Alzheimer’s Disease” series here.
This BU Today story was written by Barbara Moran.