The Lehman Lab
Research
Our overarching goal is to understand all aspects of thin filament regulation of cardiac muscle contractile function by characterizing the system at a fundamental structural level. Likewise, we aim to use our structural tools, including cryogenic electron microscopy and computational biochemistry protocols that we developed to define the root causes and potential severity of mutation based-thin filament-linked dysfunction that lead to cardiomyopathy.
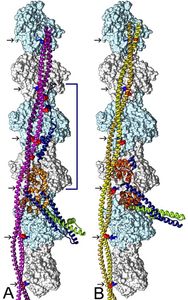
The long-held paradigm that troponin-regulated tropomyosin movement on actin of thin filaments controls myosin-head access to actin-binding sites and thus striated muscle activity was first formulated by Hanson, Lowy, and Huxley, and then supported by EM-reconstructions generated by my laboratory together with my colleagues Vibert and Craig. Recently, it has also become clear that regulated release of myosin-heads off of thick filaments and onto thin filaments augments tight control of muscle activity, increasing the need to formulate how myosin-head recruitment impacts thin filament troponin-tropomyosin and their dynamics.
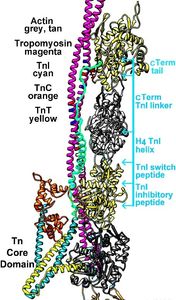
Ostensibly benign point mutations associated with cardiac muscle thin filament-linked proteins often cause subtle localized perturbation of filament structure that elicits significant hyper- or hypo-contractile behavior which over years can lead to cardiomyopathies. For example, many cases of hypertrophic (HCM), dilated (DCM), restrictive (RCM), and non-compaction (LVNC) cardiomyopathy are linked to point mutations in cardiac specific proteins that are our focus, viz. actin, myosin, troponin and tropomyosin. Indeed, our studies directly address regulation of cardiac muscle contractility at a fundamental molecular level where such disease-related “insults” may first originate and then lead to higher order dysfunction through diverse and still incompletely understood pathways. Given this complexity, it is essential to elucidate underlying structural changes and thus define early intervention strategies to prevent or delay disease progression, and later treat overt effects.
We take a “basic-science” approach while cognizant of the potential of our work to develop approaches to combat disease.
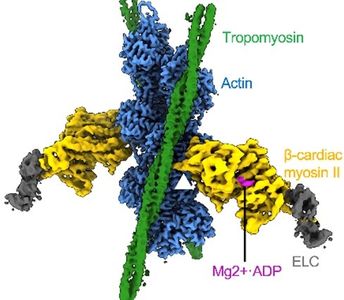
The rapidly accelerating progress in structural biology, driven by advances in cryo-EM and by the power of computational chemistry, has led to what is coined the “Resolution Revolution”; indeed, the cryo-EM of Yamada, Fujii and Namba confirmed the canonical steric model of thin filament regulation in remarkable detail. Most significantly, the possibility of precisely defining the dynamics of thin filament regulatory states at near-atomic scale is now accessible. Taking advantage of our expertise in cryo-EM and computational modeling, we published multiple papers that lay the foundation for our and colleagues future work. In particular, our studies highlight changing residue-to-residue contacts made by thin filament components with each other and myosin.
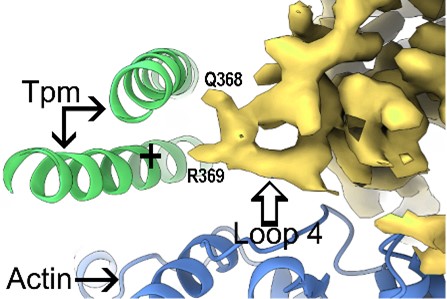
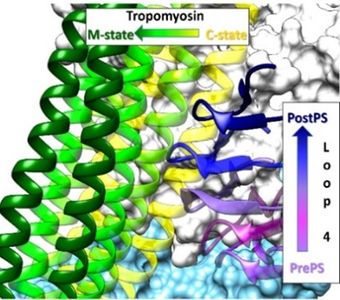
As well-positioned participants in the Resolution Revolution in structural biology, we take a lead in producing new paradigms revealing how cardiac thin filament regulation works at an atomic level and how disease-bearing mutants inexorably can lead to cardiomyopathy.
Recent Publications:
1. Creso, J.G., Gokhan, I., Rynkiewicz, M., Lehman, W., Campbell, S., Moore, J.R. (2024) In silico and in vitro models reveal the molecular mechanisms of hypocontractility caused by TPM1 M8R. Frontiers Physio. 15 doi: 10.3389/fphys.2024.1452509. PMCID not available yet.
2. Halder, S., Rynkiewicz, M.J., Kim, L., Barry, M.E., Zied, A.G.A., Sewanan, L.R., Kirk, J.A., Moore, J.R., Lehman, W., Campbell, S.G. (2025) Distinct mechanisms drive divergent phenotypes in hypertrophic and dilated cardiomyopathy associated TPM1 variants. J. Clin. Invest. Oct 22:e179135. doi: 10.1172/JCI179135. Online ahead of print. PMCID not available yet.
3. Rynkiewicz, M.J., Childers, M.C., Karpicheva, O.E., Regnier, M, Geeves, M.A., Lehman, W. (2024) Myosin’s powerstroke transitions define atomic scale movement of cardiac thin filament tropomyosin. J. Gen. Physiol. 156: e202413538. doi: 10.1085/jgp.202413538. PMCID not available yet.
4. Barry, M.E., Rynkiewicz, M.J., Pavadai, E., Viana A, Lehman W, Moore JR. (2024) Glutamate 139 of tropomyosin is critical for cardiac thin filament blocked-state stabilization. J. Mol. Cell Cardiol. S0022-2828(24)00004-X. doi: 10.1016/j.yjmcc.2024.01.004. PMCID not available yet.
5. Lehman, W., Rynkiewicz M.J. (2023) Troponin-I-induced tropomyosin pivoting defines thin-filament function in relaxed and active muscle. J. Gen. Physiol. 155:e202313421. doi: 10.1085/jgp.202313387. PMCID: PMC10227645
6. Halder, S., Howland, H.L., Creso, J., Lehman, W.J., Moore, J.R., Rynkiewicz, M.J., Sewanan, L., Campbell, S.G. (2023) Mechanisms of pathogenicity in the hypertrophic cardiomyopathy doi: 10.1093/pnasnexus/pgad011. PMCID: PMC9991458
7. Doran, M.H., Rynkiewicz, M.J., Rasicci, D., Bodt, S.M.L, Barry, M., Bullitt, E., Yengo, C.M., Moore, J.R., Lehman, W. (2023) Conformational changes linked to ADP release from human cardiac myosin bound to actin-tropomyosin. J. Gen. Physiol. 155: e202213274. doi: 10.1085/jgp.202213267. PMCID: PMC9859928
8. Campbell, S.G., Moore, J.R., Rynkiewicz, M.J., Lehman, W. (2023) Multi-scale modeling will unravel connections between sarcomeric mutations and cardiomyopathies. J. Mol. Cell. Cardio. Plus. 2023 3: 100025. No PMCID provided
9. Doran, M.H., Rynkiewicz, M.J., Pavadai, E., Bodt, S.M.L, Rasicci, D., Moore, J.R., Yengo, C.M., Bullitt, E., Lehman, W. (2023) Myosin loop-4 is critical for optimal tropomyosin repositioning on actin during muscle activation and relaxation. J. Gen. Physiol. 155: e202213274. doi: org/10.1085/jgp.202213274. PMCID: PMC9723511
10. Rynkiewicz, M.J., Pavadai, E., Lehman, W. (2022) Modeling human cardiac thin filament structures. Front. Physiol. 13:932333. doi: 10.3389/fphys.2022.932333. PMCID: PMC9257132
11. Pavadai, E., Rynkiewicz, M.J., Yang, Z., Gould, I.R., Marston, S.B., Lehman, W. (2022) Modulation of cardiac thin filament structure by phosphorylated troponin-I analyzed by protein-protein docking and molecular dynamics simulation. Arch. Biochem. Biophys. 725:109282. doi: 10.1016/j.abb.2022.109282. PMCID: PMC10680062
12. Suphamungmee, W., Lehman, W., Morgan, K.M. (2022) Functional remodeling of the contractile smooth muscle cell cortex, a provocative concept, supported by direct visualization of cortical remodeling. Biology 11:662. doi: 10.3390/biology11050662. doi: 10.1016/j.xcrm.2021.100501.PMCID: PMC9138025
13. Teekakirikul, P., Zhu, W., Xu, X. …, Rynkiewicz, M.J., Lehman, W., … Lo, C.W. (2022) Genetic resiliency associated with dominant lethal TPM1 mutation causing atrial septal defect with high heritability. Cell Reports – Medicine 3:100501. doi: 10.1016/j.xcrm.2021.100501PMCID: PMC8861813
14. Doran MH, Lehman W. The Central Role of the F-Actin Surface in Myosin Force Generation. Biology (Basel). 2021 Nov 23;10(12):1221. doi: 10.3390/biology10121221. PMCID: PMC8698748
15. Lehman W, Pavadai E, Rynkiewicz MJ. C-terminal troponin-I residues trap tropomyosin in the muscle thin filament blocked-state. Biochem Biophys Res Commun. 2021 Apr 30;551:27-32. doi: 10.1016/j.bbrc.2021.03.010. PMCID: PMC8026701
16. Sewanan LR, Park J, Rynkiewicz MJ, Racca AW, Papoutsidakis N, Schwan J, Jacoby DL, Moore JR, Lehman W, Qyang Y, Campbell SG. Loss of crossbridge inhibition drives pathological cardiac hypertrophy in patients harboring the TPM1 E192K mutation J Gen Physiol. 2021 Sep 6;153(9):e202012640. doi: 10.1085/jgp.202012640.PMCID: PMC8321830
17. Racca AW, Rynkiewicz MJ, LaFave N, Ghosh A, Lehman W, Moore JR. M8R tropomyosin disrupts actin-binding and filament regulation. The beginning affects the middle and the end. J Biol Chem. 2020; 295(50):17128-17137. doi: 10.1074/jbc.RA120.014713. PMID: 33020181
18. Doran MH, Pavadai E, Rynkiewicz MJ, Walklate J, Bullitt E, Moore JR, Regnier M, Geeves MA, Lehman W. Cryo-EM and molecular docking shows myosin loop 4 contacts actin and tropomyosin on thin filaments. Biophys J. 2020; 119(4) 821-830. doi: 10.1016/j.bpj.2020.07.006 PMCID: PMC7451941
19. Pavadai E, Lehman W, Rynkiewicz MJ. Protein-protein docking reveals dynamic interactions of tropomyosin on actin filaments. Biophys J. 2020; 119(1):77-86. doi.org/10.1016/j.bpj.2020.05.017 PMCID: PMC7335911
20. Pavadai E, Rynkiewicz MJ, Ghosh A, Lehman W. Docking troponin T onto the tropomyosin overlapping domain of thin filaments. Biophys J. 2020;118(2):325‐336. doi:10.1016/j.bpj.2019.11.3393 PMCID: PMC6976810
21. Sundar S, Rynkiewicz MJ, Ghosh A, Lehman W, Moore JR. Cardiomyopathy mutation alters end-to-end junction of tropomyosin and reduces calcium sensitivity. Biophys J. 2020;118(2):303‐312. doi:10.1016/j.bpj.2019.11.3396 PMCID: PMC6976805
22. Viswanathan MC, Schmidt W, Franz P, Rynkiewicz MJ, Newhard CS, Madan A, Lehman W, Swank DM, Preller M, Cammarato A. A role for actin flexibility in thin filament-mediated contractile regulation and myopathy. Nat Commun. 2020;11(1):2417. doi:10.1038/s41467-020-15922-5 PMCID: PMC7229152
23. Lehman W, Rynkiewicz MJ, Moore JR. A new twist on tropomyosin binding to actin filaments: perspectives on thin filament function, assembly and biomechanics. J Muscle Res Cell Motil. 2020;41(1):23‐38. doi:10.1007/s10974-019-09501-5 PMCID: PMC6697252
24. Lehman W, Maéda Y. Introducing a special issue of the Journal of Muscle Research and Cell Motility on actin and actin-binding proteins. J Muscle Res Cell Motil. 2020;41(1):1‐2. doi:10.1007/s10974-019-09569-z, PMID: 31865487, PMCID: not provided by journal.
25. Lehman W, Rynkiewicz MJ, Moore JR. A new twist on tropomyosin binding to actin filaments: perspectives on thin filament function, assembly and biomechanics. J Muscle Res Cell Motil. 2020;41(1):23‐38. doi:10.1007/s10974-019-09501-5 PMCID: PMC6697252
Earlier publications are listed on: https://www.ncbi.nlm.nih.gov/myncbi/william.lehman.1/bibliography/public/
We collaborate with colleagues in several laboratories with similar interests including:
Dr. Aaron Beeler, Department of Chemistry, Boston University.
Dr. Lauren Brown, Department of Chemistry, Boston University.
Dr. Stuart Campbell, School of Engineering and Applied Science, Yale University.
Dr. Anthony Cammarato, Department of Medicine, Johns Hopkins University.
Dr. Roger Craig, Department of Cell Biology, University of Massachusetts Medical School.
Dr. Vitold Galkin, Department of Physiological Sciences, Old Dominion University.
Dr. Michael Geeves, School of BioSciences, University of Kent, Canterbury, UK.
Dr. Malcolm Irving, Randall Centre of Cell & Molecular Biophysics, King’s College-London, UK.
Dr. Steven B. Marston, National Heart and Lung Institute, Imperial College, London, UK.
Dr. Jeffrey Moore, Department of Biological Sciences, University of Massachusetts-Lowell.
Dr. Kathleen Morgan, Department of Health Sciences, Sargent College, Boston University.
Dr. Michael Regnier, Department of Bioengineering, University of Washington.
Links:
Contact Us
William J. Lehman
Department of Pharmacology, Physiology & Biophysics
Chobanian & Avedisian School of Medicine
700 Albany Street, W408E
Boston MA 02118-2526
Phone:(617) 358-8484
e-mail: wlehman@bu.edu