The Herscovitz Lab
Research
Assembly and secretion of very low density lipoproteins (VLDL)
Our research focuses on the mechanisms that regulate the assembly of very low density lipoproteins (VLDL) in the liver and their secretion into the circulation. Apolipoprotein (apo) B, a key player in this complex process is a very large (4536 amino acids and molecular mass of about 550 kDa), hydrophobic glycoprotein that directs the assembly of VLDL. VLDL particles are remodeled in the plasma to give rise to low density lipoproteins (LDL), the major carriers of plasma cholesterol. LDL are also a major risk factor for the premature development of coronary heart disease, the leading cause of death in developed countries.
Our goal is to elucidate the molecular details of the folding of apoB into its mature, secretion-competent form. This might allow us to develop means to modulate the secretion of VLDL and thereby, reduce plasma cholesterol levels. We are working on 2 major areas as described below.
A) Chaperone-assisted folding of apolipoprotein B
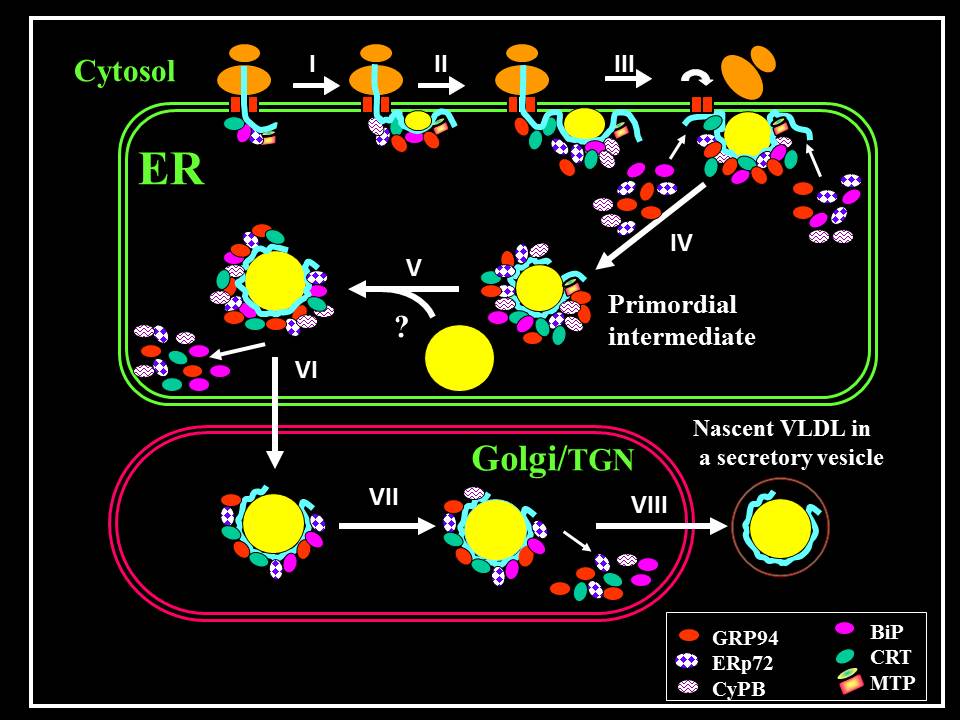
ApoB has unusual structural properties, as it is virtually water insoluble, and it requires association with lipids to attain its mature, secretion-competent form. Therefore, its maturation is more complex than typical secretory proteins. While typical nascent secretory proteins are translocated into the endoplasmic reticulum (ER) lumen where their folding and maturation take place, the folding of apoB is more complex and occurs in two major steps (see Figure). The first occurs cotranslationally while apoB is still membrane-bound. It involves partial lipidation of apoB to form “primordial” particles which are released into the ER lumen where additional folding steps occur. In the second step these particles fuse with lipid droplets rich in triacylglycerols (TAG) to form nascent VLDL. This incompletely understood step occurs either in the ER or in a post ER compartment. Only a fraction of nascent apoB is secreted. The size of this fraction is dependent on lipid availability. The underlipidated fraction is targeted for proteasome-dependent degradation (not shown).
Folding of nascent proteins in the cell is a complex process that requires the assistance of molecular chaperones, which are highly abundant conserved proteins found in all types of cells from bacteria to humans. The primary role of molecular chaperones is to bind transiently to nascent polypeptides, prevent their aggregation, (which may otherwise occur in the cell due to exposed hydrophobic domains), and maintain them in conformations competent for efficient folding. Our objective is to identify and characterize molecular chaperones that assist in the folding of apoB.
Several ER resident chaperones were identified by other investigators. Microsomal triglyceride transfer protein (MTP), BiP, calnexin and calreticilulin. MTP is unique to apoB and is critical for VLDL assembly and secretion. We identified a number of additional chaperones including GRP94, ERp72 and cyclophilin B, that interact with apoB during its maturation. We demonstrated that these chaperones interact with apoB both co- and post-translationally and that they have distinct functions during various steps of apoB maturation to form VLDL (see Figure).
Through the use of biochemical, immunological, cell and molecular biological techniques we hope to gain a better understanding of the mechanism by which molecular chaperones mediate folding of nascent proteins in general, and apoB in particular. Our long-term goal is to identify novel chaperones that may be unique to apoB and thus may serve as a target to modulate its secretion.
Zhang, J. and Herscovitz, H. 2003. Nascent lipidated Apolipoprotein B Is Transported to the Golgi as an Incompletely Folded Intermediate as Probed by Its Association with Network of Endoplasmic Reticulum Molecular Chaperones, GRP94, ERp72, BiP, Calreticulin and Cyclophilin B. J. Biol. Chem. 278 (9) 7459-7468.
B) Regulation of VLDL assembly by factors involved in lipid metabolism
Neutral lipids such as triacylglycerols (TAG) play a critical role in VLDL assembly. This process serves to export excess TAG from the liver to prevent accumulation of fat in the liver (steatosis) that damages the liver. TAG are directed to the secretory pathway by MTP and become available for VLDL assembly. We are interested in elucidating the mechanisms by which factors that regulate TAG levels also regulate VLDL assembly. To that end we reconstitute lipoprotein assembly in nonlipoprtoein secreting cells. These cells do not express apoB or MTP. They are therefore transfected to express apoB and MTP in combination with factors that regulate the level of neutral lipids such as transcription factors, SREBP 1 and 2 (sterol regulatory element binding proteins), and fatty acid binding proteins. We found that these factors increase the level and activity of ectopically expressed MTP leading to increased secretion of apoB-containing lipoproteins. Having developed this model, we can now elucidate the mechanisms involved in these effects and identify factors that can modulate them to reduce VLDL secretion.
Selected Publications
Herscovitz H., Hadzopoulou-Cladaras M., Walsh M., Cladaras C., Zannis V. I. and Small D. M. 1991. Expression, secretion, and lipid-binding characterization of the N-terminal 17% of apolipoprotein B. Proc. Natl. Acad. Sci. USA 88, 7313-7317. PMID: 1871138 PMCID: PMC52285
Herscovitz H., Gantz D., Tercyak A. M., Zannis V. I. and Small D. M. 1992
Expression of human apolipoprotein E but not that of apolipoprotein A-I by mouse C127 cells is associated with increased secretion of lipids in the form of vesicles and discs. J. Lipid Res. 33, 791-803. PMID: 1512507
Herscovitz, H., Kritis, A., Talianidis, I., Zanni, E., Zannis, V. I., and Small, D. M. 1995. Murine mammary-derived cells secrete the N-terminal 41% of human apolipoprotein B on high density lipoprotein-sized lipoproteins containing a triacylglycerol-rich core. Proc. Natl. Acad. Sci. USA, 92, 659-663. PMID: 7846033 PMCID: PMC42679
Linnik K. M. and Herscovitz H.1995. Multiple molecular chaperones interact with apolipoprotein B during its maturation. The network of endoplasmic reticulum-resident chaperones (ERp72, GRP94, calreticulin, and BiP) interacts with apolipoprotein b regardless of its lipidation state. 1998. J. Biol. Chem. 273, 21368-21373. PMID: 9694898
Burch W.L.,and Herscovitz, H. 2000. Disulfide bonds are required for folding and secretion of apolipoprotein B regardless of its lipidation state. J. Biol. Chem 275, 16267-16274. PMID: 10747912
Carraway, M., Herscovitz, H., Zannis, V.I., and Small, D.M. 2000. Specificity of lipid incorporation is determined by sequences in the N-terminal 37 of apoB. Biochemistry, 39, 9737-9745. PMID: 10933790
Herscovitz, H., Derksen, A., Walsh, M.T., McKnight, C.J., Gantz, D., Hadzopoulou-Cladaras, M. Zannis, V.I., Curry, C., and Small, D.M. 2000. The N-terminal 17% of apoB binds tightly and irreversibly to emulsions modeling nascent very low density lipoproteins. J. Lipid Res. 42, 51-59. PMID: 11160365
Zhang, J., and Herscovitz H. 2003. Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B. J. Biol. Chem. 278, 7459-7468. PMID: 12397072
Sommer, U., Herscovitz, H., Welty, F.K., and Costello, C.E. 2006. LC-MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J Lipid Res. 47, 804-814. PMID: 16443931
Links:
BU Profile
ResearchGate
PubMed
Contact Us
Department of Pharmacology, Physiology & Biophysics
Chobanian & Avedisian School of Medicine
700 Albany Street, W330B
Boston MA 02118-2526
Phone: (617) 358-8490
e-mail: haya@bu.edu