The Gursky Lab

Welcome to the Gursky Lab at Boston University School of Medicine! Our research focuses on the structural and biophysical mechanisms of lipoprotein metabolism and protein misfolding. Using techniques such as cryo-electron microscopy, spectroscopy, and computational modeling, we aim to uncover how these molecular processes contribute to cardiovascular disease and amyloidosis. Through interdisciplinary and collaborative research, we strive to advance both fundamental understanding and translational applications.
Last Updated: Aug 2025
 Crowns are our lab photo tradition!
Crowns are our lab photo tradition!Research
Free energy barriers in lipoprotein stability and remodeling
Plasma lipoproteins are nanoparticles containing several proteins and several hundred lipids, which mediate lipid transport and metabolism and are essential in cardiovascular health and disease. We uncovered that all major lipoprotein classes including high-, low- and very-low-density lipoproteins (a.k.a. Good and Bad Cholesterol) are stabilized by high free energy barriers. We developed biophysical approach to measure these barriers. By using circular dichroism spectroscopy, turbidity, electron microscopy, gel filtration and biochemical methods, we demonstrated that these barriers involve lipoprotein fusion and protein dissociation similar to those involved in metabolic lipoprotein remodeling. Studies of lipoprotein stability pioneered by our lab helped obtain relative rates of remodeling of lipoprotein classes and subclasses and assess how various in vivo factors can modulate these rates. One example is current kinetic studies of aggregation and fusion of low-density lipoproteins, which are thought to trigger atherosclerosis.
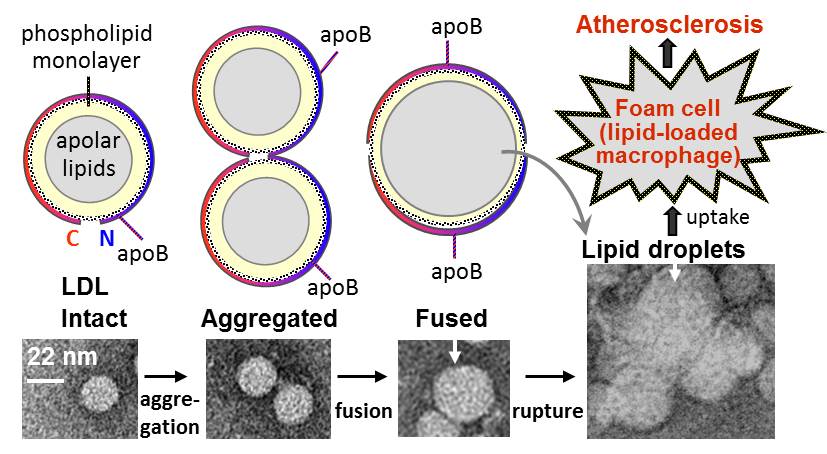
Research by Mengxiao Lu, PhD in Biophysics 2014 Lu M, Gantz DL, Herscovitz H, Gursky O.
J Lipid Res. 2012; 53(10):2175-2185 Biomol Concepts. 2013; 4(5):501-518
Protein misfolding and amyloid
Our studies have revealed novel aspects of misfolding and aggregation of amyloid-forming proteins. We were the first to report heat-induced beta-sheet folding and aggregation in amyloid-beta peptide and to demonstrate kinetic control in the misfolding and aggregation of immunoglobulin light chain. We proposed the molecular mechanisms for misfolding and aggregation of naturally occurring mutants of apolipoproteins A-I and A-II that cause amyloid disease in humans. Our approach combines circular dichroism and fluorescence spectroscopy, turbidity, calorimetry, electron microscopy with biochemical and immunochemical methods and structural and bioinformatic approaches. Local, national and international collaborations provide invaluable expertise in other methods of structural and cell biology and in translational research.
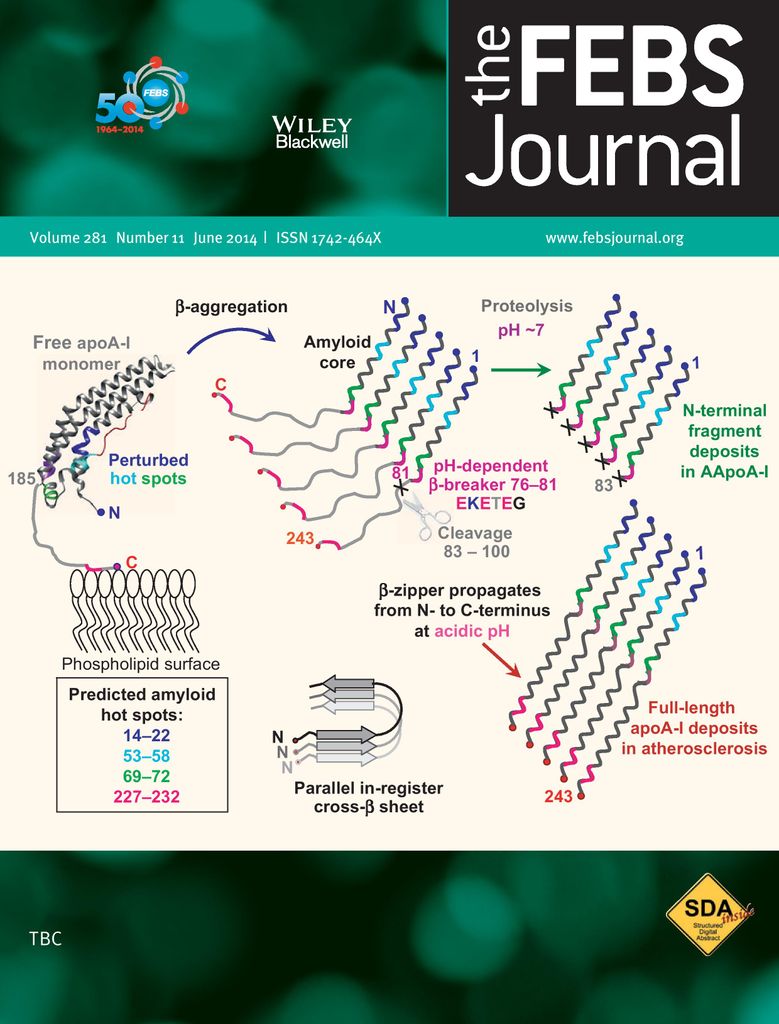
Amyloidogenic mutations in human apolipoprotein A-I are not necessarily destabilizing — a common mechanism of apoA-I misfolding in familial amyloidosis and atherosclerosis. Das M, Mei X, Jayaraman S, Atkinson D, Gursky O.
FEBS J. 2014; 281(11):2525–2542
Recently, we investigated the structural mechanisms underlying amyloid light-chain (AL) amyloidosis, a protein misfolding disorder driven by aggregation of monoclonal immunoglobulin light chains (LCs). Using high-resolution cryogenic electron microscopy (cryo-EM), we resolved the fibril architecture of cardiac AL-224L amyloid belonging to the overrepresented λ6 LC family.
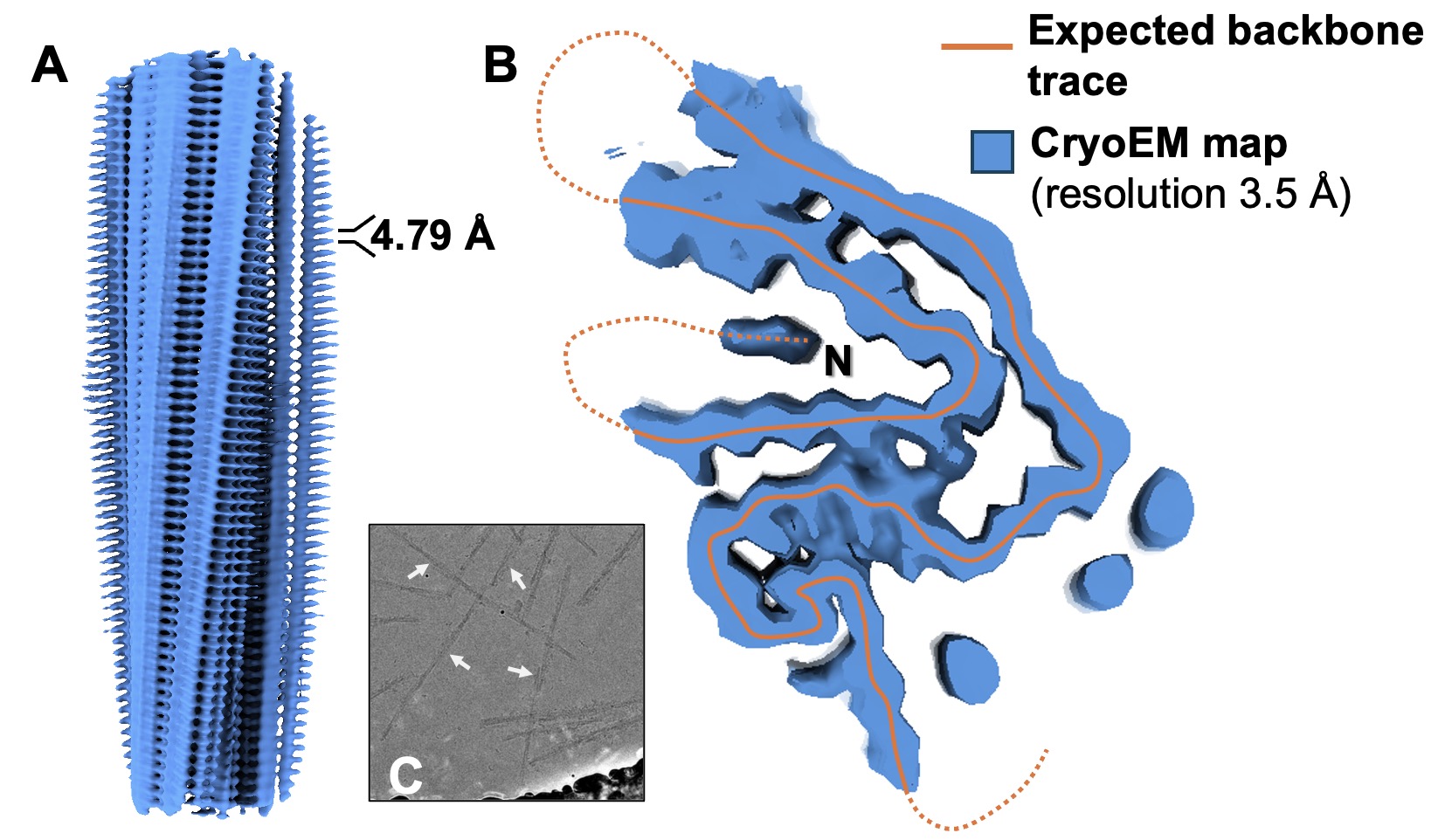
Cryo-EM structure of AL252L fibrils derived from liver tissue by Noorul Huda, PhD. (A) Side view of the AL252L fibril cryo-EM density map at 3.5 Å resolution showing characteristic 4.79 Å helical rise of β-strands along the fibril axis. (B) Cross-sectional view of the density map (blue) with the expected backbone trace (orange), highlighting disordered region with dotted line.
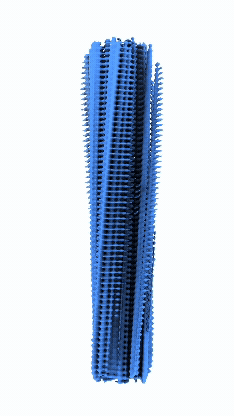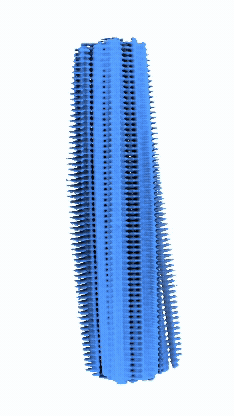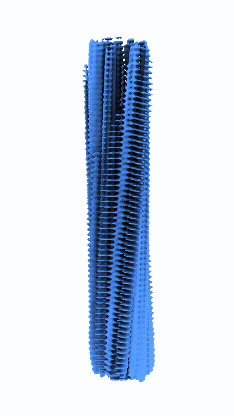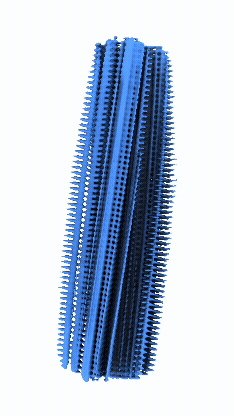

Micrographs/Exposures representing fibrils of Serum Amyloid A with CSA
Dr. Gursky has authored more than 50 peer-reviewed journal articles and several book chapters (click here for Pubmed list). She is an elected Fellow of the American Heart Association (AHA), an editorial board member of the Journal of Lipid Research, and a frequent reviewer for NIH, NSF, AHA and other national, international and private agencies. She serves as a member of the Biophysics of Biological Membranes (BBM) study section at NIH. She is an editor of a book “Lipids in Protein Misfolding” in preparation for publication by Springer in 2015. She has served as a visiting professor at the Indian Institute of Technology (IIT) Bombay. She is teaching major PhD-level courses and is involved in graduate admissions and mentoring.
Dr. Gursky has mentored a number of pre- and postdoctoral trainees who went on to their own successful careers in life sciences. All past PhD students graduated in 5 to 5 ½ years with a couple of first-author publications. We have a track record of maintaining a family-friendly environment and attracting women in science.
The Group
- Olga Gursky, PhD – Principal Investigator, Professor
- Shobini Jayaraman, PhD – Senior Scientist
- Noorul Huda, PhD – Postdoctoral Researcher
- Kyeongseo Choi – Undergraduate RA/Webmaster
Collaborations
Dr. Haya Herscovitz – BUSM Physiology & Biophysics. Apolipoprotein cell biology.
Dr. David Atkinson – BUSM Physiology & Biophysics. ApoA-I mutants.
Dr. Catherine E. Costello – BUSM Mass Spectrometry Resource. Ion mobility MS.
Dr. John R. Engen – Northeastern University. H-D exchange mass spectrometry.
Dr. Marcus Fändrich – University of Ulm, Germany. Serum Amyloid A.
Dr. José Luis Sánchez-Quesada – Int. de Recerca, Barcelona Spain. Electronegative LDL
Lab Alumni
Emily Lewkowicz, PhD
Madhurima Das, PhD
Isabel Morgado, PhD
Donald Gantz
Publications
- An unusual phenotype of hereditary AApoAI amyloidosis caused by a novel Asp20Tyr substitution is linked to pH-dependent aggregation of apolipoprotein AI
T Prokaeva, S Jayaraman, E Klimtchuk, N Burke, B Spencer, D Nedelkov, O Gursky
Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, 2025 - Serum Amyloid A Binding to Glycosaminoglycans is Synergistic with Amyloid Formation: Therapeutic Targeting in the Inflammation-linked Amyloidosis
S Jayaraman, A Urdaneta, M Fandrich, O Gursky
Journal of Molecular Biology, 2025 - Cryo-EM of cardiac AL-224L amyloid reveals shared features in λ6 light chain fibril folds
CW Hicks, T Prokaeva, B Spencer, S Jayaraman, N Huda, S Wong, O Gursky
bioRxiv, 2025 - Elucidating the Mechanism of Recognition and Binding of Heparin to Amyloid Fibrils of Serum Amyloid A
CB Abraham, E Lewkowicz, O Gursky, JE Straub
Biochemistry, 2024 - Conformational differences in the light chain constant domain of immunoglobulin G and free light chain may influence proteolysis in AL amyloidosis
ES Klimtchuk, T Prokaeva, BH Spencer, S Wong, S Ghosh, A Urdaneta, O Gursky
Journal of Molecular Biology, 2024 - Science around the world
A Plaza-Florido, O Gursky, ML Herrera, CD Moschopoulos, Y Sohrabi
Trends in Molecular Medicine, 2024 - Amyloid and collagen templates in aortic valve calcification
S Jayaraman, N Narula, J Narula, O Gursky
Trends in Molecular Medicine, 2024
Teaching
GMS FC711 Foundations in Biomedical Sciences I: Protein Structure, Catalysis and Interaction The second module of the Foundations in Biomedical Science course provides students with a quantitative understanding of protein structure, function, posttranslational modifications and the turnover of proteins in the cell. In addition, students gain facility with thermodynamics, catalysis, kinetics and binding equilibria.
FC708 Professional Development Skills The objective of this course is to extend students’ education beyond the traditional biomedical course content so as to enable students to develop critical professional skills. Consideration is given to presentation skills, issues of compliance/ethics & the law as well as personal professional development. The course draws on a wide variety of experts throughout the university.
GMS BY 763 Foundations of Biophysics and Structural Biology II This graduate level course provides a thorough grounding in the theory and major experimental methods of Biophysics and Structural Biology. The course covers thermodynamic and spectroscopic methods, computational biology and structural NMR.
GMS BY 871, 872 Biophysics Seminar This is a special topics seminar series for first and second year graduate students. Each student presents several papers per semester describing the background, the specific methods, the results, the conclusions of the authors, and a critique of the work.