Programmed Problem Set on Cardiovascular Drugs
W. Mark Vogel, Ph.D.
Adjunct Associate Professor of Pharmacology
Boston University School of Medicine
Questions or comments should be mailed to Carol Walsh
Return to Pharmacology Problem Sets
This problem set will:
- familiarize you with hemodynamic measurements used to evaluate cardiovascular drugs
- review the pharmacology of digitalis glycosides
- demonstrate how initial hemodynamic status determines the net effect of direct and reflex drug actions
Before solving these problems review the text and lecture notes on digitalis and other inotropic agents. Table 1 in this problem set lists some normal hemodynamic values, to help you evaluate the clinical data; please don’t try to memorize them.
| Table 1. Some normal hemodynamic values |
| Heart Rate (beats/min) |
60-100 |
| Arterial Systolic Pressure (mmHg) |
100-140 |
| Arterial Diastolic Pressure (mmHg) |
60-90 |
| Mean Arterial Pressure (mmHg) |
70-105 |
| Left Ventricular End Diastolic Pressure (mmHg) |
3-12 |
| Mean Right Atrial Pressure (mmHg) |
2-10 |
| Pulmonary Capillary Wedge Pressure (mmHg) |
2-10 |
| Left Ventricular P/t (mmHg/sec) |
1000-2000 |
| Cardiac Output (L/min) |
varies with patient’s size |
| Cardiac Index (L/min/m^2) |
2.6-4.2 |
| Stroke Volume Index (ml/m^2/beat) |
30-65 |
| Systemic Vascular Resistance (dyne€sec€cm^5) |
700-1600 |
| Systemic Vascular Resistance (mmHg/L/min/m^2) |
17-40 |
Figure 1 shows the effects of digitalis administered on five consecutive days to a 33 year old man with rheumatic heart disease (Stewart et al., Arch. Int. Med. 62:569, 1938).
Answer the next five items based on the data in Figure 1.
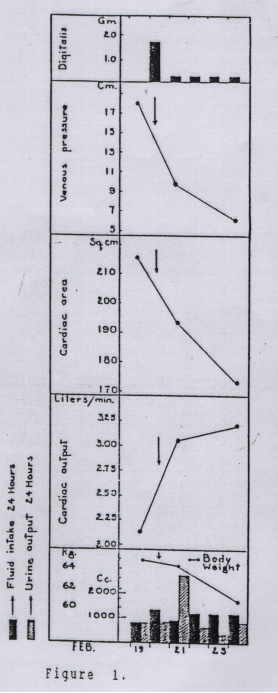
I. Why were the subsequent doses of digitalis smaller than the first dose?
a. Toxicity developed after the first dose.
No, there is no evidence of toxicity in this figure; return to Item I and try another response.
b. Volume of distribution decreased after the first dose due to the decrease in body weight.
No, this small weight loss would not alter the volume of distribution significantly. Return to Item I and try another response.
c. Due to its long plasma half life, digitalis is often administered as a large loading dose, followed by smaller maintenance doses.
Yes, remember it takes 4 to 7 half-lives to reach steady state plasma levels with multiple dosing. Proceed to Item II.
d. Due to its short plasma half life, digitalis is often administered as a large loading dose, followed by smaller maintenance doses.
No, digitalis glycosides have relatively long half lives. A loading dose is often used because it takes 4 to 7 half lives to reach steady state plasma concentrations with multiple dosing. Return to Item I and try another response.
II. What best explains the decrease in cardiac area seen on chest X-rays after digitalis?
a. Digitalis decreased heart size by decreasing venous return.
No, digitalis can decrease venous return in subjects with normal heart function by sequestering blood in the liver. But in this patient with heart failure, cardiac output increased. Thus, venous return must have increased (what comes out must = what goes in). There must be another explanation for the decrease in venous pressure. Return to Item II and choose again.
b. Digitalis decreased heart size by increasing ejection fraction.
Correct, now try Item III.
c. Digitalis decreased heart size by increasing cardiac diastolic tone.
No. That is the explanation the author offered for the decrease in heart size but there is a better explanation. Return to Item II and make another selection.
d. Digitalis decreased heart size by decreasing circulating plasma volume.
No. Digitalis would decrease plasma volume after several days (by reversing salt and water retention) but that does not explain the decrease in heart size. Venous return must increase in order for cardiac output to increase. Return to Item II and make another choice.
III. What best explains the decrease in venous pressure after digitalis?
a. Digitalis decreased circulating plasma volume.
Yes. Decreased circulating plasma volume in only part of the explanation. Increased ejection fraction also contributes to the decrease in venous pressure because decreased ventricular chamber volume and diastolic pressure result in decreased venous filling pressure. Go on to Item IV.
b. Digitalis increased ejection fraction, thus decreasing left ventricular diastolic volume and pressure, with a secondary decrease in venous pressure.
Yes. The improvement in ejection fraction is only part of the explanation. A decrease in circulating plasma volume also contributes to the decrease in venous pressure. Go on to Item IV.
c. Both a and b above contribute to the decrease in venous pressure.
Correct, proceed to Item IV.
d. Digitalis is a direct venodilator.
No, digitalis is a venoconstrictor. Return to Item III and make another selection.
IV. What best explains the decrease in body weight after digitalis?
a. Digitalis caused a diuresis.
Yes, diuresis explains the decrease in plasma volume. Go on the Item V.
b. Digitalis caused nausea and anorexia.
No. A toxic concentration of digitalis can cause nausea and anorexia but there is a better explanation for the decrease in body weight. Go back to Item IV and make another response.
c. The slight decrease in body weight is probably unrelated to administration of digitalis.
No, digitalis caused the weight loss. Go back to Item IVc and try again.
d. Digitalis decreased appetite by a direct effect on the CNS medullary nuclei that control appetite.
No. Digitalis has no direct effect on appetite, although toxic concentrations do cause nausea and anorexia. Find a better response to Item IV.
V. What best explains the increase in urine output after digitalis?
a. Digitalis inhibited Na/K ATPase in the distal tubule of the kidney.
No. Digitalis can inhibit renal Na/K ATPase, but this is not the primary reason for the diuresis. Go back to Item V and make another selection.
b. Digitalis increased fluid intake by a direct effect on the CNS medullary nuclei that control thirst.
No. Digitalis has no particular effect on regulation of thirst, nor was fluid intake significantly increased. Go back to Item V and make another choice.
c. Digitalis caused an increase in sympathetic tone which decreased renin production by the juxtaglomerular apparatus.
No, an increase in sympathetic tone would increase renin production by a beta-1 adrenergic effect. Go back to Item V and try another response.
d. Digitalis decreased renin and aldosterone production by improving perfusion of the kidney.
Correct, go on to Item VI.
The next five items will be based on Tables 2 and 3, which describe the effects of digitalis in subjects with normal or failing hearts. Data in Table 2 are from studies by Seltzer et al. (Br. Heart J. 21:335, 1959; Circulation 25: 695, 1962). The 15 patients with heart failure were treated with sodium restriction and diuretics but no digitalis for a month before the study. They were asymptomatic upon ordinary activity. The patients with heart failure included several with hypertensive heart disease. Baseline hemodynamic values were measured 30 minutes after right heart catheterization. Post-drug values were measured 50 minutes after intravenous administration of 1.25 to 2.0 mg of digoxin.
Table 3 shows data from a similar study by Mason and Braunwald (J. Clin. Invest. 43: 532, 1964). All 6 heart failure patients were in New York Heart Association Class III or IV, none were treated with diuretics. In addition to systemic hemodynamics, forearm blood flow was measured by plethysmography. After right heart catheterization and baseline measurements, 0.5 to 0.6 mg of ouabain were administered intravenously and responses measured an hour later.
Table 2. (data from Seltzer et al)
Effects of digoxin in subjects with normal or failing heart |
|
ARTERIAL PRESSURES |
HEART
RATE
(bpm) |
CARDIAC
INDEX
(L/min/m^2) |
SYSTEMIC
RESISTANCE
(MAP/CI) |
SYST.
(mmHg) |
DIAST.
(mmHg) |
PULSE
(mmHg) |
MEAN
(mmHg) |
| Normal no Rx |
124
±4 |
78
±4 |
46
±4 |
94
±3 |
81
±2 |
3.1
±0.2 |
31
±2 |
| +Digoxin |
132
±5 |
79
±5 |
54*
±2 |
96
±5 |
77*
±3 |
2.9
±0.2 |
36*
±4 |
|
| Failure no Rx |
146
±10 |
88
±6 |
59
±6 |
107
±7 |
84
±4 |
2.0
±0.1 |
56
±4 |
| +Digoxin |
158*
±10 |
86
±6 |
72*
±7 |
110
±7 |
78
±3 |
2.5*
±0.1 |
47*
±4 |
Values are means ±SEM. * indicates significant effect of digoxin (p < 0.05) by paired t-test.
SYST = systolic, DIAST = diastolic, MAP = mean arterial pressure, CI = cardiac index.
Table 3. (data from Mason and Braunwald)
Hemodynamic effects of ouabain in subjects with normal or failing hearts |
|
MAP
(mmHg) |
FBF
(ml/min/100g) |
FVR
(MAP/FBF) |
HR
(bpm) |
CI
(L/min/m^2) |
SVI
(ml/beat/m^2) |
SVR
(MAP/CI) |
| Normal no Rx |
83
±3 |
3.1
±2 |
36
±0.4 |
69
±2 |
3.4
±0.1 |
49
±3 |
25
±1 |
| +Ouabain |
92*
±4 |
2.9*
±0.3 |
35*
±4 |
63*
±2 |
3.3
±0.1 |
53*
±2 |
28*
±1 |
|
| Failure no Rx |
80
±2 |
1.7
±0.2 |
52
±6 |
108
±8 |
1.7
±0.2 |
15
±1 |
51
±6 |
| +Ouabain |
79
±3 |
2.2*
±0.3 |
40*
±5 |
87*
±7 |
2.7*
±0.3 |
32*
±3 |
30*
±3 |
Values are means ±SEM. * indicates significant effect of ouabain (p < 0.05) by paired t-test.
MAP = mean arterial pressure, FBF = forearm blood flow, FVR = forearm vascular resistance, HR = heart rate, CI = cardiac index, SVI = stroke volume index, SVR = systemic vascular resistance.
In the following items “digitalis” will be used to refer to either digoxin or ouabain.
VI. Is there any evidence that digitalis exerted a positive inotropic effect in the two groups of normal subjects?
a. No, one would not expect digitalis to have a positive inotropic effect in a normal, non- failing, heart.
No. Digitalis has a positive inotropic effect on the nonfailing heart; see if you can find evidence for it and make another response to Item VI.
b. No, in fact, the effects of cardiac index and forearm blood flow suggest that digitalis exerted a negative inotropic effect.
No. Digitalis did not significantly change cardiac index. The decreased forearm blood flow is due to increased vascular resistance. Cardiac contractility was increased. Return to Item VI and find the evidence.
c. Yes, as indicated by the effects on mean arterial pressure.
No. Mean arterial pressure increased significantly in only one of the two normal groups. An increase of arterial pressure can result from increased vascular resistance and does not necessarily indicate an inotropic effect. Return to Item VI and choose again.
d. Yes, as indicated by the effects on arterial pulse pressure and stroke volume index.
Yes, both effects imply increased cardiac contractility, especially increased stroke volume despite increased vascular resistance. These are indirect indicators of contractility; other studies have directly measured increased force of contraction by normal myocardium after digitalis. Go on to Item VII.
VII. What best explains the modest decrease in heart rate in normal and heart failure patients after digitalis?
a. Digitalis directly depresses sinus node automaticity.
No. Digitalis directly decreases sinus automaticity only at high, toxic, concentrations. There is a better explanation, so make another selection for Item VII.
b. Digitalis slows conduction through the AV node, mainly by increasing parasympathetic (vagal) tone.
No. The statement is true but does not explain the decreased heart rate. This effect of digitalis leads to an increased PR interval, or eventually to AV conduction block. Return to Item VII and make another choice.
c. Digitalis stimulates parasympathetic (vagal) tome and, particularly in patients with heart failure, causes reflex withdrawal of sympathetic tone.
Correct, now go to Item VIII.
d. All of the above contribute to the effect of digitalis on heart rate.
No, only one choice is correct. Go back to Item VII and try again.
VIII. In the Mason and Braunwald study (see Table 3) the heart failure patients had a high baseline heart rate and a large decrease in heart rate after digitalis. Of the 6 patients, 4 had atrial fibrillation, which often accompanies heart failure. Does some effect of digitalis specifically decrease ventricular rate in patients with atrial fibrillation?
a. Yes, digitalis increases the refractory period and slows conduction through the AV node.
Correct; slower A-V conduction results in fewer rapid atrial depolarizations being conducted to the ventricles. Go on to Item IX.
b. Yes, digitalis usually converts atrial fibrillation to normal sinus rhythm.
No. Digitalis occasionally has this effect but there is another effect of digitalis that consistently slows ventricular rate in patients with atrial fibrillation. Go back to item VIII and identify that effect.
c. Yes, digitalis directly decreases atrial automaticity.
No, the response is wrong on several counts. Atrial fibrillation is probably due to aberrant conduction (re-entry or circus conduction), so altered atrial automaticity would not affect the arrhythmia. If anything, toxic concentrations of digitalis would induce abnormal automaticity in atrial cells. Atrial cells do not normally have the property of automaticity; it is normally present only in specialized pacemaker and conduction cells. Go back to Item VIII and make another choice.
d. No, the greater decreases of heart rate in these patients was probably related to the higher initial heart rate.
No. The fall in heart rate after digitalis is greater when initial heart rate is higher. However, there is a more specific action of digitalis in patients with atrial fibrillation. Return to Item VIII and try to identify the action.
IX. Why did cardiac index and forearm blood flow increase after digitalis in patients with heart failure, but not in patients with normal hearts?
a. Mean arterial pressure increased to a greater extent after digitalis in patients with heart failure.
No. Mean arterial pressure did not increase significantly in the patients with heart failure. Return to Item IX and make another selection.
b. Digitalis increased sympathetic tone and vascular resistance in normal subjects, but decreased vascular resistance in heart failure patients by withdrawal of sympathetic tone.
No. You’ve almost got it, but the increase in vascular resistance in subjects with normal hearts is due to a direct vascular constrictor effect of digitalis, not due to increased sympathetic tone. Go back to Item IX and look for a better answer.
c. Digitalis increased vascular resistance in normal subjects by a direct vascular effect, but decreased vascular resistance in heart failure patients by withdrawal of sympathetic tone.
Correct, the decrease in sympathetic tone in patients with heart failure is probably due to the increase in arterial pulse pressure, since mean arterial pressure did not increase significantly. Now go on to Item X.
d. Digitalis increases cardiac contractility in failing myocardium but not in normal myocardium.
No. Digitalis does increase contractility of normal myocardium. You may want to review Item VI before you return to Item IX and make another response.
X. In the study by Seltzer et al. (see Table 2) pulmonary capillary wedge pressure was measured in 7 of the patients with heart failure. Pulmonary capillary wedge pressure decreased from a baseline value of 24±4 mmHg to 16±3 mmHg after digoxin. What is the significance of a pulmonary capillary wedge pressure over 20 mmHg?
a. A pulmonary capillary wedge pressure over 20 mmHg suggests the presence of pulmonary edema.
Correct, at capillary pressure above 20 mmHg, hydrostatic pressure in the capillary begins to exceed plasma oncotic pressure and fluid is forced from the vasculature into the lungs. Go on to XI.
b. A pulmonary capillary wedge pressure above the normal range of 2 - 10 mmHg suggests the presence of right ventricular failure.
No, high central venous pressure and peripheral edema would indicate right heart failure. Return to Item X and choose another answer.
c. A pulmonary capillary wedge pressure over 20 mmHg indicates the presence of pulmonary hypertension (cor pulmonale).
No, pulmonary hypertension would be indicated by a high right ventricular systolic pressure over 30 mmHg. Go back to Item X and make another choice.
d. A pulmonary capillary wedge pressure over 20 mmHg suggests that these patients had only mild heart failure.
No, a pulmonary capillary wedge pressure over 20 mmHg indicates severe heart failure with pulmonary edema. Go back to Item X (hint: try answer a).
The remaining items refer to clinical studies of WIN47203, an investigational drug for treating heart failure. In animal studies, WIN47203 had positive inotropic activity, did not inhibit Na/K ATPase, and was not an adrenergic agonist. Maskin et al. studied WIN47203 in 11 patients with severe heart failure (Circulation 67: 1065, 1983). Hemodynamic values measured before and after administration of WIN47203 are presented in Table 4 and Figure 2.
Table 4. Hemodynamic effects of WIN47203
in patients with heart failure |
|
HR
(beats/min) |
MAP
(mmHg) |
CI
(L/min/m^2) |
PCWP
(mmHg) |
SVR
(dyne€sec€cm^-5) |
| Baseline |
90±4 |
75±2 |
1.9±0.1 |
27±3 |
1590±120 |
| +WIN47203 |
88±4 |
72±2* |
2.9±0.1* |
16±2* |
1070±90* |
Values are means ±SEM. * indicates significant effect of drug (p < 0.05) by paired t-test.
HR = heart rate; MAP = mean arterial pressure; CI = cardiac index; PCWP = pulmonary capillary wedge pressure; SVR = systemic vascular resistance.
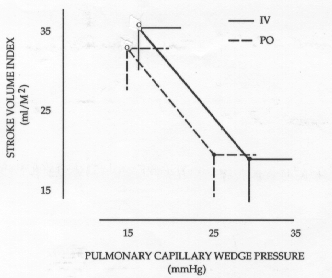
Figure 2. Stroke volume index and pulmonary capillary wedge pressure in 11 patients during the control period (closed circles) and at maximum response (open circles) after i.v. and oral WIN 47203. Values are means ±SD.
XI. Which of the baseline values in Table 4 indicate that the patients are in heart failure?
a. High pulmonary capillary wedge pressure.
True, a pulmonary capillary wedge pressure over 12 mmHg is abnormal and over 20 mmHg suggests the presence of pulmonary edema. These patients have additional hemodynamic evidence of heart failure. But consider other answer choices; go back to Item XI.
b. Low cardiac index.
True, a cardiac index lower than 2.5 L/min/m^2 is evidence of heart failure. These patients have additional hemodynamic evidence of heart failure. Go back to Item XI and look for another correct answer.
c. High systemic vascular resistance.
True, the average systemic vascular resistance for this group of patients is at the upper limit of the normal range, suggesting a high sympathetic tone. But there are other hemodynamic abnormalities in these patients. Go back to Item XI and look for another correct answer.
d. All of the above.
Correct; these patients have a high pulmonary capillary wedge pressure, low cardiac index, and high systemic vascular resistance; all are indicators of heart failure. Heart rate is somewhat high but within the normal range. Mean arterial pressure is normal because baroreceptor reflexes keep arterial pressure constant. Now, go on to Item XII.
XII. What symptomatic benefit might result from the hemodynamic effects of WIN47203 in patients with heart failure?
a. Increased cardiac index might decrease symptoms of fatigue.
True, increased cardiac index should improve skeletal muscle perfusion and decrease fatigue. You may have forgotten, however, that decreased pulmonary capillary pressure will produce an added benefit of decreased congestive symptoms. Go to Item XIII.
b. Decreased pulmonary capillary pressure might decrease congestive symptoms (e.g. dyspnea).
True, decreased pulmonary capillary pressure will relieve congestive symptoms. You forgot that increased cardiac index will improve skeletal muscle perfusion and decrease symptoms of fatigue. Now go on to Item XIII.
c. Both a and b above.
Correct, go on to Item XIII.
d. The drug acts only as a vasodilator and, thus, would not diminish symptoms of heart failure.
Yes. Arteriolar vasodilation can relieve symptoms of fatigue by increasing cardiac output. Venodilation can relieve congestive symptoms by decreasing cardiac filling pressure. Go to Item XIII to see if this drug is a vasodilator.
XIII. From the hemodynamic data above, what appears to be the primary action of WIN47203 in patients with heart failure.
a. It acts as an arteriolar vasodilator.
No. WIN47203 has some arteriolar dilator activity; mean arterial pressure and systemic vascular resistance were both decreased, with an increase in cardiac output. However, it must have an additional action because pure arteriolar dilators do not cause large decreases in pulmonary capillary wedge pressure. Go back to Item XIII and make another selection.
b. It acts as a mixed arteriolar and venous/capacitance vasodilator.
True, a mixed arteriolar/venous vasodilator can increase cardiac index (as a result of decreased systemic resistance) and can decrease pulmonary capillary wedge pressure (as a result of venous pooling and decreased filling pressure). The Starling curve would be shifted upward and to the left as in Figure 2. But, remember, an inotropic agent can cause similar results by increasing ejection fraction (which decreases ventricular diastolic pressure), and secondarily by decreasing vascular resistance by withdrawal of sympathetic tone (like digitalis). WIN47203 must have some vasodilator activity, because mean arterial pressure was decreased, unlike a pure inotropic effect which would leave arterial pressure unchanged or slightly increased. To see if the drug has inotropic activity, go on to Item XIV.
c. It acts as a positive inotropic agent with a reflex decrease in systemic vascular resistance.
Yes. Ionotropic agents can decrease pulmonary capillary wedge pressure by increasing ejection fraction (thus decreasing ventricular diastolic pressure), and can decrease vascular resistance by withdrawal of sympathetic tone (like digitalis). A mixed arteriolar/venous vasodilator can produce the same result, increasing cardiac index by decreasing systemic resistance, and decreasing pulmonary capillary pressure due to venous pooling and decreased filling pressure. The Starling curve would be shifted upward and to the left as in Figure 2. The drug is a vasodilator because it decreased mean arterial pressure, unlike a pure inotropic agents, which would leave arterial pressure unchanged or slightly increased. To see if the drug has inotropic activity, go to Item XIV.
d. The drug could act by any one or a combination of 1 - 3 above; additional data are needed to distinguish these possibilities.
Correct; for more explanation, read the comment to Item XIIIc before going on to Item XIV.
To determine if WIN47203 has a clinically important cardiac inotropic effect, Ludmer et al. (Circulation 73: 130, 1986) compared the effects of drug vehicle (5% dextrose in water, D5W), drug administered intravenously, and drug administered directly into the left main coronary ostium. They reasoned that effects of intracoronary administration would be due to direct actions on the heart, while effects of intravenous administration would be due to both cardiac and vascular effects. Plasma drug concentration was 372±50 ng/ml after intravenous administration. Intracoronary infusion achieved a similar coronary plasma concentration with a negligible systemic concentration of 52 ng/ml. The results of this experiment are presented in Figures 3 and 4.
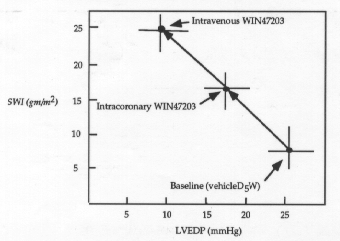
Figure 3. Stroke volume index (SWI) as a function of left ventriclar end diastolic pressure (LVEDP)
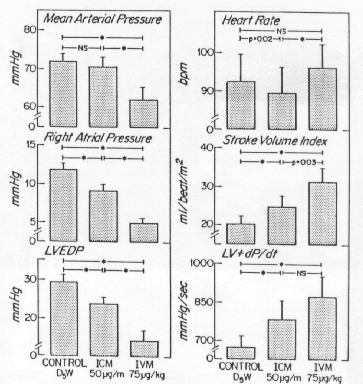
Figure 4. Comparative hemodynamic effects of vehicle (D5W), intracoronary infusion of WIN47203 at 50µg/min (ICM), and intravenous infusion of WIN47203 (IVM) in eight consecutive patients. * = p < 0.01
XIV. What do you conclude about the action of WIN47203 in patients with heart failure from the data in Figures 3 and 4.
a. The drug acts primarily as a vasodilator with very little direct inotropic effect on the heart.
No, intracoronary drug administration had no effect on mean arterial pressure but it increased LV+dP/dt and stroke volume while decreasing filling pressures. This indicates that the drug had some direct inotropic activity. Return to Item XIV and choose again.
b. The drug acts primarily as a positive inotropic agent with a reflex decrease in vascular resistance similar to that produced by digitalis.
You conclude correctly that direct positive inotropic activity contributes to the effects of this drug (intracoronary administration increased both stroke volume and LV +dP/dt). The decrease in heart rate probably results from decreased sympathetic tone, secondary to the inotropic effect. However, the decrease in mean arterial pressure after intravenous administration suggests that the drug has vasodilator activity. A pure positive inotropic drug would not decrease mean arterial pressure. Thus, both cardiac and vascular actions contribute to the hemodynamic effects of this drug, which we now know as milrinone. Congratulations, you have completed the problem set!
c. The drug has both significant inotropic activity and direct vasodilator activity.
Correct, a direct inotropic effect is indicated by the increase of LV+dP/dt and stroke volume during intracoronary administration. That resulted in decreased filling pressures and a reflex decrease in heart rate. The decrease in arterial pressure after systemic administration of the drug indicates that the drug also has a direct arteriolar vasodilator action. WIN47203 is now known as milrinone. Congratulations, you have completed the problem set!
d. The drug has relatively pure venous capacitance dilator activity, like that produced by organic nitrates
No, the effect of intracoronary administration shows that the drug has direct effects on the heart. Go back to Item XIV and choose another answer.



