Hui Feng, M.D., Ph.D.
Associate Professor of Pharmacology, Physiology & Biophysics and Medicine
Section of Hematology and Medical Oncology
Research Interests:
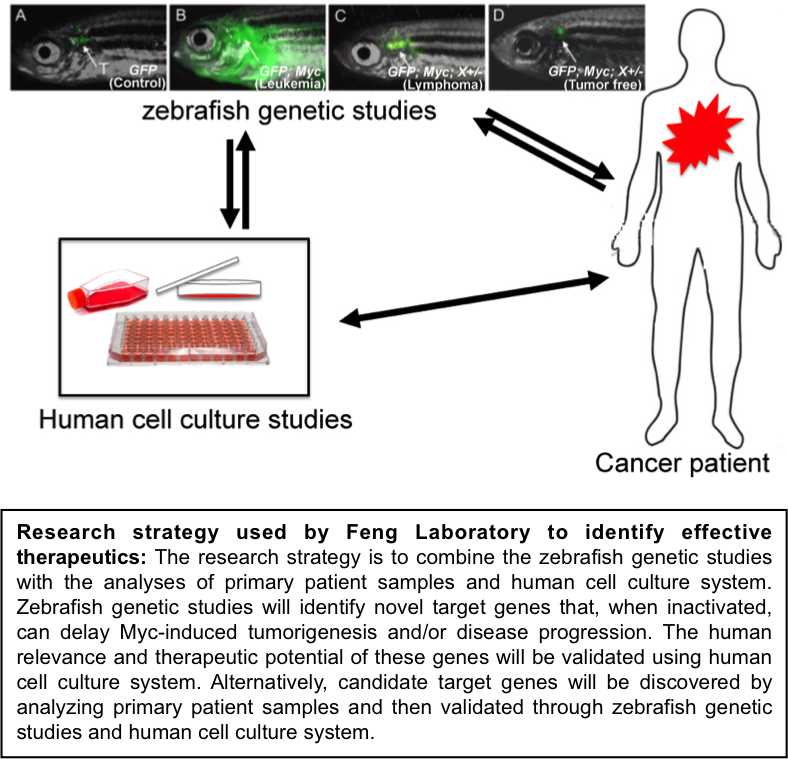
Dr. Feng is Director of the Laboratory of Zebrafish Genetics and Cancer Therapeutics. Dr. Feng’s research interests focus on identifying novel genes and pathways that are essential for MYC/MYCN-driven tumor initiation and progression, particularly for T-Lymphoblastic Lymphoma/Leukemia, Breast Cancer, and Neuroblastoma. The research strategy of Dr. Feng’s research is to combine the analysis of human cancer genomic databases with the genetic and imaging capacities of the zebrafish system.
Current research:
Cancer takes the lives of millions each year and over 90% of cancer-related deaths result from tumor metastasis. Therefore; there is an urgent need to develop novel strategies that can block tumor metastasis and kill cancer cells efficiently and specifically. MYC, aptly referred to by Gerard Evan as the oncogene (cancer-causing gene) from hell, is aberrantly activated in nearly all human cancers, including leukemias, lymphomas, neuroblastoma, and carcinomas. Cancers with aberrant MYCactivity are often aggressive, rapidly spreading to distant tissues. These facts underscore the need to improve understanding of the regulatory molecules involved with MYC-induced tumor initiation and progression, thus allowing the researchers to discover and exploit the vulnerabilities of the cancers, in order to develop novel and effective drugs targeting MYC-related cancers.
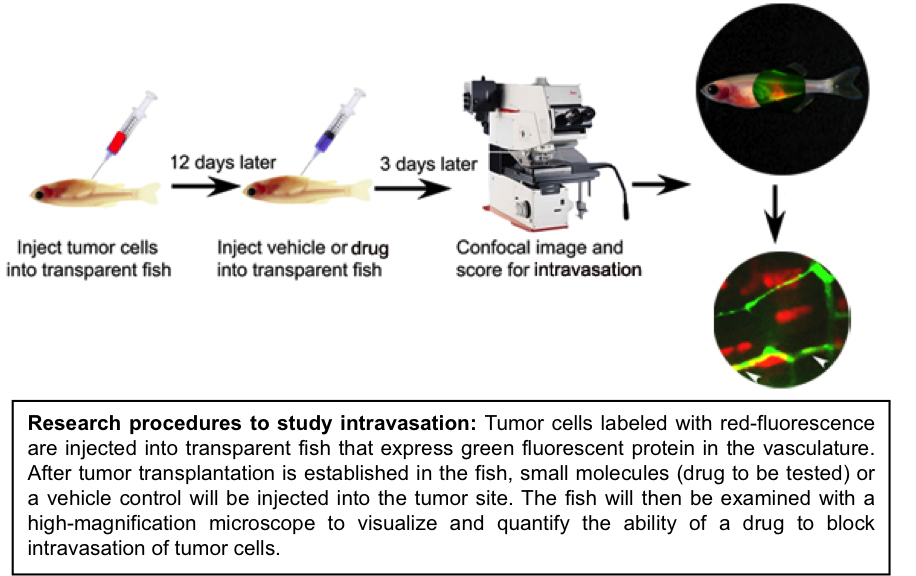
The zebrafish offers many unique advantages as a cancer model: easy monitoring of tumor development in vivo, due to its transparency and the ability to differentially fluorochrome-label tumor cells and vasculature; a high degree of genetic similarities with humans; simple techniques for functional studies of candidate genes identified through ongoing human genomic analysis; and the feasibility of conducting genetic and chemical screens to dissect the molecular pathways of tumorigenesis and identify promising lead compounds. The research in Feng laboratory uses the zebrafish model in combination with human cell line and patient sample analyses,
1) to determine the molecular mechanisms underlying tumor cell intravasation and tumor progression.
2) to identify genes and pathways that, when inactivated, delay the initiation and progression of MYC-related cancers.
3) to test the identified genes’ therapeutic potential in treating MYC-related cancers and to characterize their molecular relevance to MYC.
One major project in Feng laboratory is to dissect molecular pathways that regulate the initial step of tumor metastasis – intravasation – the entry of tumor cells into vascular and/or lymphatic vessels. Improved understanding of tumor cell intravasation is the key for future therapeutic developments to block the spread of tumor cells from their primary site. Through interrogating human cancer genomic information with the imaging and genetic capacities of the zebrafish system, researchers in Feng’s laboratory focuses on identifying novel “intravasation genes” that promote the intravasation of MYC–driven tumor cells. Using this combined approach, the Feng laboratory has successfully identified multiple promising genes. Genes identified through these studies should represent promising targets for therapeutic intervention to block MYC-associated metastasis. As the next step of the research, their therapeutic potential will be evaluated using human cancer cell lines.
The second major project in Feng’s laboratory is to exploit the genetic capacity of the zebrafish system to identify genes that, when inactivated, can inhibit or delay tumor development. Researchers in Feng laboratory have identified a gene critical for energy production and macromolecule synthesis – ~50% reduction of this gene delayed Myc-induced T-cell leukemia but did not influence fish development. This finding is consistent with MYC’s important role in cancer metabolism.
The long-term goal of Dr. Feng’s research is to discover novel molecular therapies to target critical components of MYC-driven oncogenic pathways, thus providing treatment alternatives that are more specific and less toxic.
Funding Support
- National Cancer Institute
- American Cancer Society
- National Science Foundation
- St. Baldrick Foundation
- Mary Kay Ash Foundation
- Leukemia Research Foundation
- Karin Grunebaum Cancer Research Foundation
- Boston University
Lab Page
Publications
Recent News