Laboratory of Genomic Instability and Cancer Therapeutics
Research
The telomere is a repetitive DNA element that protects the ends of linear chromosomes. Mammalian telomeres are bound by a ribonucleoprotein cap consisting of the protein complex shelterin, and the telomere repeat containing RNA, TERRA. Together the entire telomere complex functions to maintain genome stability. However, each time a cell replicates and divides a small stretch of telomeric DNA is lost. Telomeres that become critically short activate cell cycle checkpoints and promote the cellular senescence and/or apoptosis that drive organismal aging. Thus, while checkpoint activation promotes aging, it serves as a barrier to the development of cancer. In cancer cells, the genes governing checkpoint activation are often and as a result, critically short telomeres fail to induce cell cycle arrest. These eroded telomeres are then recognized as substrates for DNA damage repair, stimulating the genomic instability that drives cancer progression. Therefore, the Laboratory of Genome instability and Cancer therapeutics is interested in understanding how normal telomeres are maintained, how dysfunctional telomeres arise, and how defects in this process contribute to the onset of cancer. Current projects in the lab are aimed at understanding the following;
1. TERRA
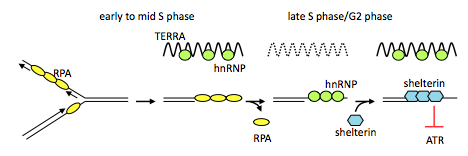
Telomere dysfunction is a major source of genomic instability contributing to the development of human cancer. The dynamics of telomere maintenance has been a mainstay of cancer research for decades, however, the discovery of the telomere repeat-containing RNA, TERRA, suggests that there is still much to learn about telomere biology. Previous studies have made significant contributions to our understanding of TERRA thus far, however, we are still eager to gain detailed mechanistic insight. TERRA is known to associate with telomeres and with a number of telomere-binding proteins. In addition, TERRA knockdown induces telomere dysfunction and instability. Despite these initial characterizations relatively little is known about how TERRA functions either on, or independent of, telomeric chromatin. We have demonstrated one of the first known function for TERRA in that the cell cycle-regulated association of TERRA with telomeres drives a molecular switch coordinating telomere replication with telomere end protection. Moving forward we are interested in further defining the role of TERRA in telomere end protection.
2. Alternative Lengthening of Telomeres (ALT)
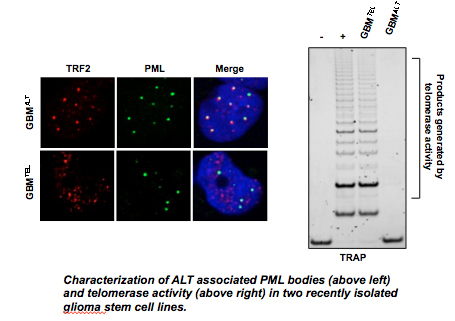
Cancer cells overcome the replicative senescence associated with critically short telomeres by exploiting mechanisms of telomere elongation. Reactivation of the enzyme telomerase, or activation of the Alternative Lengthening of Telomeres (ALT) pathway, account for cellular immortalization in approximately 95% of all human cancers. While the molecular mechanisms leading to activation of these pathways has not been fully elucidated, improperly capped telomeres are believed to be an early event in the process. Our lab is interested in not only understanding how ALT arises, but also how ALT is maintained in cancer. We have recently characterized several new ALT positive osteosarcoma and glioma stem cell lines and have begun to dissect the mechanisms required for ALT maintenance. The goal of these studies will be to identify tractable targets in the treatment of ALT positive cancers including, osteosarcoma and glioblastoma.

 Jane Lock – MD/Ph.D. Student
Jane Lock – MD/Ph.D. Student Alysia Bryll
Alysia Bryll Aneesh Patel
Aneesh Patel Kelli Cox – Ph.D. Student
Kelli Cox – Ph.D. Student