Carbohydrate Analysis by MSn: LNFP II
ESI MS3 of permethylated lacto-N-fucopentose isomer (LNFP II)
(All FT ICR data from 9.4 T ESI FT ICR at Marshall’s lab, NHMFL)
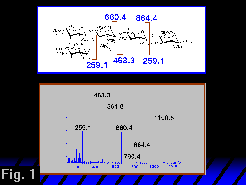
The ion fragmentation spectrum of natriated and permethylated LNFP II obtained from a triple quadrupole mass spectrometer is shown in Figure 1 (click on thumbnail link for full image).
The parent ion is a doubly charged molecule carrying two sodium cations at m/z 561.8 (singly natriated molecular ion at m/z 1100.5). The SORI CID spectrum of the singly natriated LNFP II obtained by ESI FT-ICR at 9.4 tesla is shown in Figure 2 along with the triple quadrupole CID spectrum for comparison. 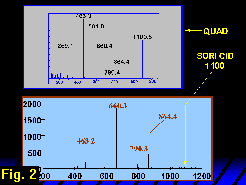
The m/z value 561.8 is consistent with a composition of deoxyhexose (dHex), three hexoses (Hex) and an N-acetylhexosamine (HexNAc). In general, two features of the oligosaccharide’s structure may be addressed by tandem mass spectrometry. The first and most accessible feature is the sequence and branching structure, or, more accurately, the connection topology, which is obtained from glycosidic cleavages. The second feature is the glycosidic linkage positions (linkage analysis), which may be inferred from particular cross-ring cleavages. Anomeric configurations and monosaccharide identification are only rarely obtained from tandem MS.
The well-known tendency of HexNac residues to direct fragmentation to their reducing side and the prominent fragment ions at m/z 463 and 660 would be expected of a B/Y fragment pair arising from a glycosidic cleavage on the reducing side of the HexNAc residue and carrying a single sodium cation.
In the triple quadrupole mass spectrum (Figure 1) the m/z 660 and 463 fragment pair is incremented and decremented (respectively) by 204 u, forming a pair at m/z 864 and 259. We could therefore infer another glycosidic B/Y pair, indicating a methylated Hex residue on the reducing side of the HexNAc. In the FT-ICR SORI CID MS2 spectrum (Figure 2), the m/z 259 peak is also observed, but at low magnitude. Hence a cursory inspection of the glycosidic cleavages strongly suggests a (dHex, Hex, HexNAc)-Hex-Hex topology, in which the connection among the residues at the non-reducing end is not defined. Based on the triple quadrupole data, one would like to extend this simple topological analysis to the non-reducing terminal trisaccharide (dHex, Hex, HexNAc)- by assigning ions at m/z 259 and 229 as glycosidic non-reducing terminal Hex and dHex C1-type ions. From this assignment one would draw the conclusion that the HexNAc is a branched residue. The problem is that the glycosidic fragments on the non-reducing side of the HexNAc are C1 ions and a hexose C1 fragment at m/z 259 is isobaric with a hexose Y1 fragment. Therefore, a non-reducing terminal hexose cannot be unambiguously identified from this spectrum.
The m/z 660 ion was isolated after dissociation of the m/z 1100 parent (Figure 3) and was then dissociated in turn to form an MS3 FT-ICR mass spectrum (Figure 4) 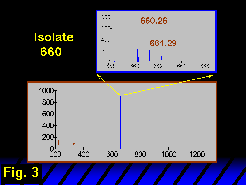 from which the m/z 259 ion may be associated unambiguously with a non-reducing terminal hexose. The glycosidic fragments at m/z 472, 259, 442 and 424 are associated with the branched topology, whereas the absence of glycosidic fragments at m/z 415, 433 or 268 argues against a linear structure.
from which the m/z 259 ion may be associated unambiguously with a non-reducing terminal hexose. The glycosidic fragments at m/z 472, 259, 442 and 424 are associated with the branched topology, whereas the absence of glycosidic fragments at m/z 415, 433 or 268 argues against a linear structure.
Moreover, a significant feature of the MS3 spectrum (Figure 4) is the appearance of fragmentation pathways not observed in the dissociation of the molecular ion (Figures 1, 2).
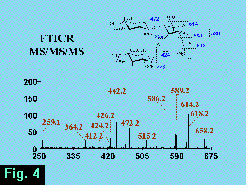 Although we have not examined linkage isomers to LNFP II by this method, it is clear that the lack of fragmentation of the non-reducing terminal trisaccharide a priori precludes identifying structural isomers of this part of the molecule by ion fragmentation analysis.
Although we have not examined linkage isomers to LNFP II by this method, it is clear that the lack of fragmentation of the non-reducing terminal trisaccharide a priori precludes identifying structural isomers of this part of the molecule by ion fragmentation analysis.