Congratulations Dr. Kingston!
Congratulations to Nathan (Varelas Lab) who successfully defended his thesis this past Wednesday (9/29/2021).

Best of luck in your future.
Congratulations to Mikel Garcia-Marcos on his promotion to Full Professor of Biochemistry!
Congratulations to Mikel Garcia-Marcos on his promotion to Full Professor of Biochemistry!

The Garcia-Marcos Lab investigates signal transduction mechanisms with the ultimate goal of elucidating the molecular basis of human diseases and developing novel therapeutic approaches.
Congratulations to Joe Zaia on his SfG Award.
Congratulations to Professor Joe Zaia who was awarded the 2021 ASBMB Molecular and Cellular Proteomics Lectureship Award by the Society for Glycobiology (SfG).

Congratulations Dr. Hicks-Berthet!
Congratulations to Julia (Varelas Lab) who successfully defended her thesis this past Thursday (7/15/2021).
Best of luck in your future. 
Research News: New insight into signals that limit goblet cell specification in the lung.
The Varelas Lab has published a study in Cell Reports describing important roles for the transcriptional regulators YAP and TAZ, which are key effectors of Hippo pathway signaling, in lung epithelial homeostasis.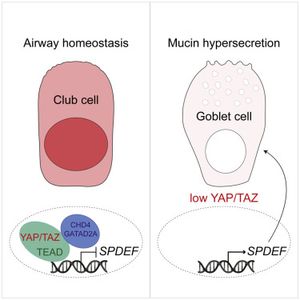
The study explored roles for YAP and TAZ in adult lung epithelium and found that conditional deletion of theYap and Wwtr1/Taz genes in animal models led to severe lung damage that included alveolar and bronchial cellular defects. One notable phenotype that was further studied was an aberrant production of mucus secreting goblet cells that resulted from the transdifferentiation of airway club cells with low YAP/TAZ activity.
Goblet cells secrete mucus that normally helps protect the lining of the bronchus and traps microorganisms for clearance. However, aberrant specification of goblet cells leads to defective lung functions that associate with a range of lung diseases (e.g. asthma, COPD, Cystic Fibrosis and chronic bronchitis). Identification of YAP/TAZ as key regulators of goblet cell production offers insight into molecular signals that may dysregulated in such diseases.
Gene expression and chromatin binding analyses from murine and human lung models revealed that YAP/TAZ repress a gene expression network that is important for controlling goblet cell production. Interestingly, altering signals that induce nuclear YAP/TAZ activity was sufficient to block goblet cell production in experimental cell models, suggesting that signals governing YAP/TAZ activity may offer a therapeutic means to restrict goblet cell production in disease.
For full text of the article, click here.
Research News: Saeed Lab Makes New SARS-CoV-2 Discoveries
The Saeed Lab, in partnership with the Broad Institute of MIT and Harvard, has published new findings in Cell.
In this study, the Saeed lab discovered SARS-CoV-2 elements being identified by human T cells to initiate an immune attack against the virus. As a background, the human immune system has two arms: innate immunity and adaptive immunity. While innate immunity launches a quick, non-specific fight against the virus, adaptive immunity is slow and utilizes specific viral components to get activated and unleash severe attack on the virus. A distinctive feature of adaptive immunity is that it “remembers” the viral identity, and if encountered by the same virus in the future, sets in motion a quick and intense campaign to overpower the virus. The adaptive immunity can be further divided into two subgroups: B cell immunity (a.k.a. humoral immunity) and T cell immunity (a.k.a. cellular immunity). While B cell immunity acts by generating antibodies against the virus, T cell immunity employs specialized cells to destroy the virus-infected cells.
Although a number of studies have reported SARS-CoV-2 elements being recognized by human B cells to produce antibodies, there remained a paucity of knowledge regarding viral components being identified by human T cells, and this is despite the fact that the role of T cells in SARS-CoV-2 clearance has been increasingly recognized. To fill this knowledge gap, the Saeed lab collaborated with a research group at the Broad Institute of MIT and Harvard and performed mass spectrometry-based analysis of viral peptides (protein fragments) that have the ability to activate T cells. This led to the discovery of 37 peptides. Strikingly, eight of these peptides originated from the hidden, non-canonical SARS-CoV-2 proteins that are often overlooked by the scientists.
The team then performed a series of experiments in human and mice to test if the identified viral peptides were capable of activating T cells. Most of them indeed were. To scientists’ surprise, some peptides derived from the hidden viral proteins provoked the strongest immune response, highlighting an unexpected yet critical role of these proteins in virus biology. These findings will change the way scientists study the human immune responses to SARS-CoV-2 and will also inform the development of next-generation vaccines. Incorporation of viral components capable of invoking strong T cell responses is expected to produce a vaccine that is more effective and broadly protective against newly emerging SARS-CoV-2 variants.
For full text of the article, click here.
Dr. Valentina Perissi becomes Co- PI and Director of the Boston Nutrition Obesity Research Center
As of June 1, 2021 Dr. Valentina Perissi has replaced Dr. Caroline Apovian as Co- PI and Director of the Boston Nutrition Obesity Research Center (BNORC). This leadership change has been approved by the Center’s Executive Committee and NIH National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK). Dr. Valentina Perissi will co-lead the Center with Dr. Andy Greenberg.
The Boston Nutrition Obesity Research Center is funded by the NIH/NIDDK. BNORC is a consortium of institutions that includes Boston Medical Center, Boston University School of Medicine, Tufts University, Jean Mayer USDA Human Nutrition Research Center on Aging, the Harvard T.H. Chan School of Public Health, and Beth Israel Deaconess Medical Center. The Center’s Cores, Pilot and Feasibility Program and Enrichment programs promote inter- and multi-disciplinary research in nutrition and obesity. The Center’s Pilot and Feasibility Program has helped support many promising early career investigators who have gone on to exceptionally successful scientific careers.
Research News: New Research will Allow Convenient Investigation of Human Innate Immune Response to Viral Infections
Check out new research out of the Lau Lab and Saeed Lab.
New Research will Allow Convenient Investigation of Human Innate Immune Response to Viral Infections
(Boston)—Researchers from Boston University School of Medicine (BUSM) report the formation of human cells containing a green fluorescent protein or GFP (one of the most important proteins in biology and fluorescence imaging) genetically fused with two interferon stimulated genes (ISGs), namely Viperin and ISG15. This new creation makes these cells highly valuable reagents for reporting innate immune responses to viral infections, including those caused by coronaviruses.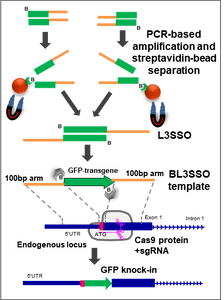
These engineered cells, which turn green when treated with interferon, are highly novel because this is the first time a reporter gene (a gene that enables the detection or measurement of gene expression), such as GFP, has been inserted into endogenous loci of ISGs.
Viral infections cause human cells to sound a biochemical alarm via interferon signaling, leading to a high-level expression of ISGs. However, ISGs are very tightly regulated genes, because too much expression and an inability to later tamp down ISG levels can be just as detrimental to cellular health.
This overactive innate immune signaling leads to a ‘cytokine storm’ (when an infection triggers the immune system to flood your bloodstream with inflammatory proteins called cytokines) that distinguishes a mild case of virus infection from fully debilitating symptoms, an outcome all too prevalent in the COVID19 pandemic. “Better research tools for studying ISG regulation are still needed, not just now for coronavirus research but for many other viruses that our society will contend with,” explained co-corresponding author Nelson Lau, PhD, associate professor of biochemistry at BUSM.
In order to successfully tag human antiviral genes with GFP, the researchers first needed to produce a new methodology for creating a long DNA repair template for more efficient and authentic CRISPR-Cas9 genome editing in animal cells. They named his template methodology the BL3SSO (sounds like “blasso”) because it sets up the DNA for more accurate repair and for introduction of a fluorescent transgene like GFP into the site cut by Cas-9 during genome editing.
These findings are the result of a collaboration between the Lau lab and the lab of Mohsan Saeed, PhD, both faculty members in BUSM’s department of biochemistry and investigators at the BU National Emerging Infectious Diseases Laboratories (NEIDL).
These findings appear online in the journal G3 : Genes | Genomes | Genetics.
This work was supported by Brandeis University Sprout Grants, the Leir Foundation, a donation from M.H.Y. Lau, Boston University start-up funds, and the National Institutes of Health via R21HD088792, R01AG052465 and R01GM135215 to NCL. MS was supported by Boston University start-up funds.
Jarrod Moore Awarded Kilachand Fellowship
Congratulations to graduate student Jarrod Moore of the Emili Lab, now a Kilachand Fellow in the Multicellular Design Program (MDP). Jarrod's research will focus on studying the pro-fibrotic cross-talk between cardiomyocytes and cardiac fibroblasts in a novel model for hypertrophic cardiomyopathy, a leading cause of sudden cardiac death in young adults. This research seeks to generate holistic molecular maps of these interactions, which will assist in discovering novel approaches for counteracting this irreversible scarring process and advancing cardiac research for these patients.
Biochemistry Russek Day Winners
The Department of Biochemistry Henry I. Russek Achievement Day Awards Committee is pleased to announce this year’s award winners. Nathan Kingston [Dr. Xaralabos (Bob) Varelas’s lab] is the first prize winner, and Joseph Kern [Dr. Xaralabos (Bob) Varelas’s lab] is the second prize winner. Our congratulations go out to the winners as well as their advisor! We look forward to hearing about their work on Henry I. Russek Achievement Day; remember it’s this week (Thursday, May 6th and Friday, May 7th) so mark your calendars for the virtual events.
The keynote address will be delivered on Thursday May 6th at 9:20 AM by Dr. Daniel Haber, Director of the MGH Cancer Center, “Circulating Tumor Cells: Insights into Cancer Metastasis”. Please go to the following site for updated information and to register so as to get the Zoom link:
https://www.bumc.bu.edu/gms/academics/phd-programs/russek-student-achievement-day/