The Perissi Lab has recently published in Pharmacological Research a paper titled “Inhibition of K63 ubiquitination by G-Protein Pathway Suppressor 2 (GPS2) regulates mitochondria-associated translation”.
The paper reports the characterization of a novel strategy for regulating mitochondria-associated translation based on the mitochondria retrograde factor GPS2 modulating non-proteolytic ubiquitination of pmitochondria-associated translation factors. Removal of GPS2, either by genomic deletion or via stress-induced translocation to the nucleus, licenses K63 ubiquitination of poly-A binding protein PABPC1 and other factors, favoring translation initiation and promoting the expression of an anti-oxidant stress response program.
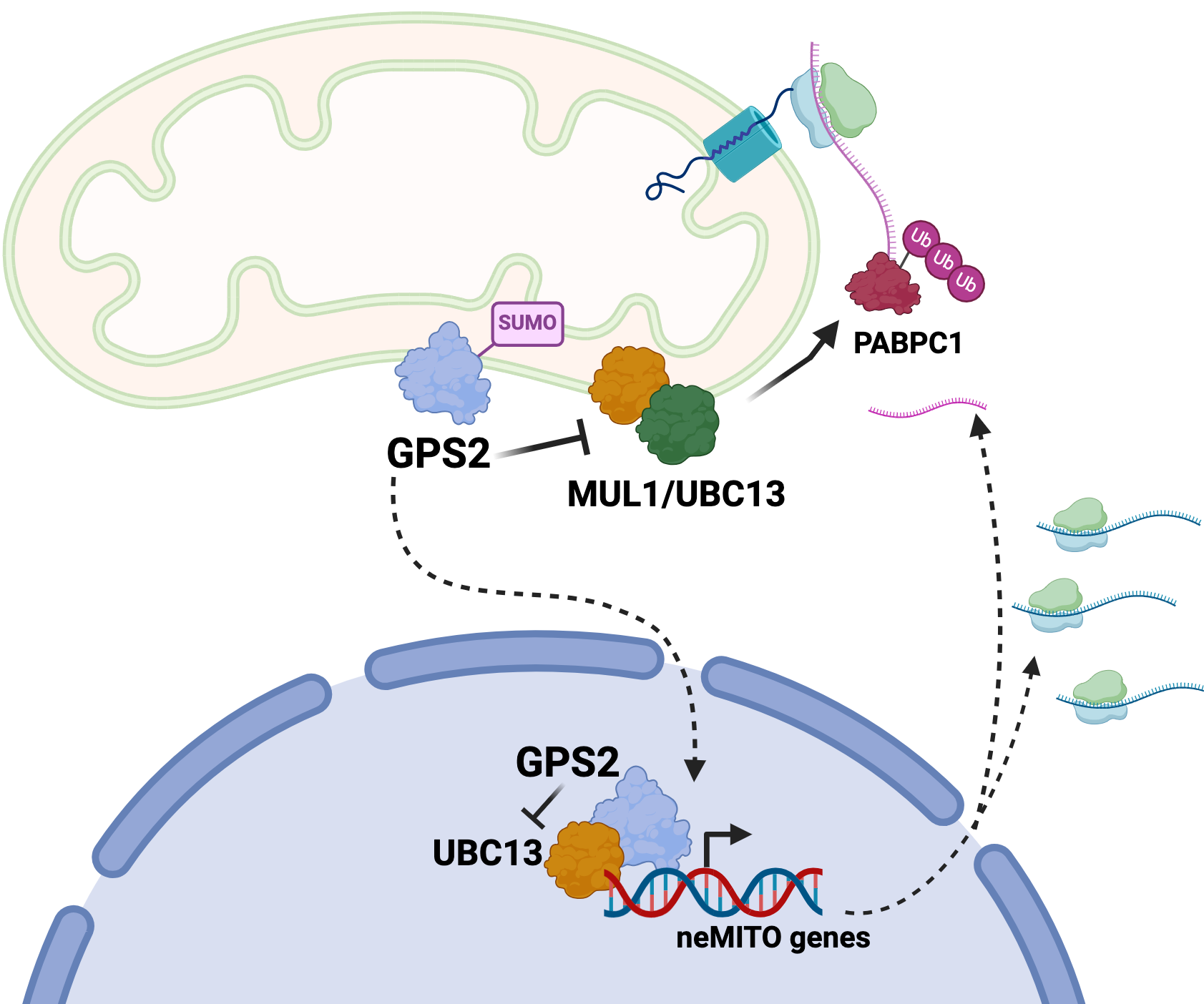
Translation of selected nuclear-encoded mitochondrial mRNAs on mitochondria-associated ribosomes provides a unique opportunity for regulating protein translation at single organelle level and thus for coordinating gene expression with mitochondrial health. These findings, together with the previous characterization of nuclear GPS2 as an essential cofactor for the expression of nuclear-encoded mitochondrial genes, reveal that GPS2-mediated inhibition of K63 ubiquitination provides a unifying strategy for modulating the expression of mitochondrial proteins through coordinated transcription and translation events.
The study was led by former postdoctoral fellow Dr. Yuan Gao, in collaboration with the Center for Network Systems Biology and the Lyons Lab.