IPCP Home
With current global HIV infection estimates exceeding 42 million people, the development of a safe, effective, and acceptable topical microbicide to prevent the sexual transmission of HIV could play a major role in world-wide reduction of the over 7,000 new HIV infections per day, potentially saving millions of lives. Topical microbicides are agents which when applied to the penis, vagina and/or the lower gastrointestinal tract tract via the rectum can result in inhibition of the transmission of HIV during sexual exposure. Microbicides may also inhibit sexually transmitted infections that may alter susceptibility to HIV infection and/or act as co-factors in HIV transmission.
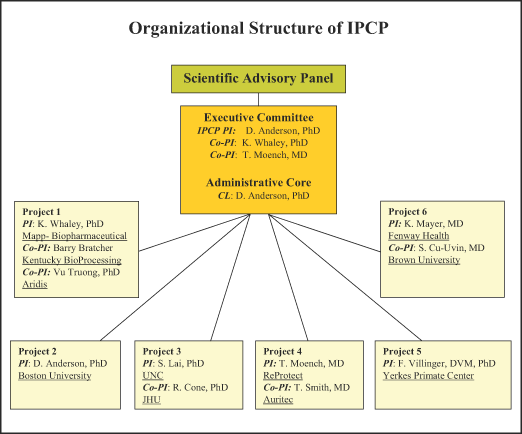
The IPCP-HTM Program of the National Institute of Allergy and Infectious Diseases at NIH has been integral in supporting the transition of topical microbicide candidates from early discovery to initial clinical testing. The IPCP-HTM Program currently supports a wide range of microbicide candidates including small chemically-defined molecules, peptides and proteins targeting a wide range of microbicide-relevant targets. Other microbicide strategies such as siRNA and bioengineered Lactobacilli are also being supported. In addition to iterative development of candidates, the IPCP-HTM Program supports research designed to advance our understanding of the mechanism(s) of sexual transmission of HIV and new technologies and approaches for assessing the safety, efficacy and acceptability of microbicide candidates.
Monoclonal Antibody-Based Multipurpose Microbicides
This IPCP-HTM brings together a network of investigators to perform multidisciplinary preclinical and exploratory clinical studies with the goal of advancing a safe, novel multi-antibody topical microbicide, mapp66. Mapp66 contains a combination of three monoclonal antibodies, produced by genetic engineering in Nicotiana benthamiana, that is designed to block distinct mechanisms of HIV sexual transmission: 1) neutralization of free HIV virions, and 2) prevention of herpes simplex virus infection, a sexually transmitted pathogen that enhances HIV infection. The monoclonal antibodies will be formulated into a biodegradable film and controlled release vaginal rings, and will be tested in vitro and in vivo for pharmacokinetics/dynamics, toxicity and anti-microbial efficacy. Antibody-based products being developed by this IPCP-HTM are an example of Multipurpose Prevention Technologies (MPTs).



