New publication from the Garcia-Marcos Lab: Inhibitory probes for spatiotemporal analysis of Gαs protein signaling
NEW PUBLICATION BY THE GARCIA-MARCOS LAB
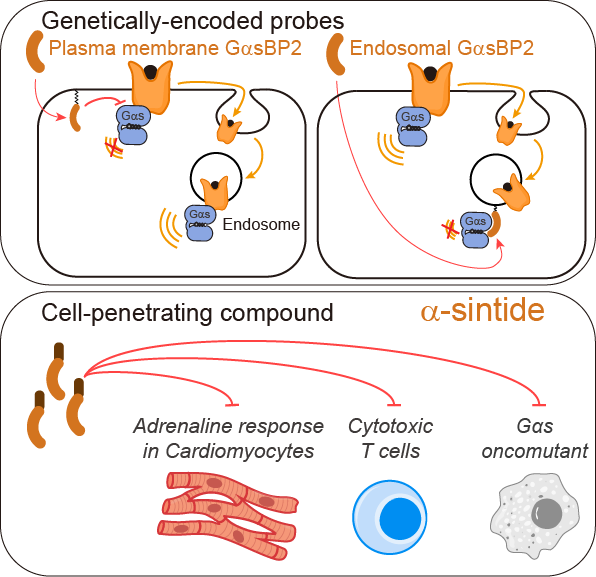
A recent publication in Nature Chemical Biology (https://www.nature.com/articles/s41589-025-02138-1) led by Jingyi Zhao in the Garcia-Marcos Lab describes the discovery and optimization of broadly applicable genetically-encoded probes and peptide-based compounds specifically inhibit Gαs, the prototypical signal transducer of G protein-coupled receptors (GPCRs). These tools were leveraged to provide new mechanistic insights into GPCR signaling at the subcellular scale by revealing definitive evidence for G protein signaling at endosomes. The newly developed peptide-based compound named α-sintide allowed the inhibition signaling in multiple contexts: blocking an oncogenic G protein mutant, inhibiting heart cell responses to adrenaline, or preventing T cell exhaustion.
This work was done in collaboration with the Varelas Lab in our Department and with the Irannejad Lab and Vilardaga Lab at UCSF and U of Pittsburgh, respectively.
Congratulations to our BU Pilot Grant Awardees!
Congratulations to our BU Pilot Grant Awardees!
Please join us in congratulating the five Biochemistry & Cell Biology PIs who were selected for BU Pilot Grants:
-
Daniel Cifuentes – Spivack Neuroscience Pilot Grant
-
Valentina Perissi – Dahod Breast Cancer Research Pilot Grant
-
Nelson Lau – Sexual Medicine Pilot Grant
-
Bob Varelas – Dahod Breast Cancer Research Pilot Grant
-
Mike Blower – Shipley Prostate Cancer Pilot Grant
Well deserved—congratulations to all!
New research from the Cifuentes Lab: RBPscan, a quantitative in vivo tool for profiling RNA-binding protein interactions.
New research from the Cifuentes Lab: RBPscan, a quantitative in vivo tool for profiling RNA-binding protein interactions.
Researchers in the Cifuentes lab at Boston University have published a new study in Molecular Cell describing RBPscan, a method that enables quantitative measurement of protein-RNA interactions directly in living cells.
RNA-binding proteins regulate nearly every step of gene expression, but existing methods have made it difficult to determine where these proteins bind and how strongly they interact with RNA in their native cellular context. RBPscan was developed to address this challenge by combining RNA editing with massively parallel reporter assays to provide a simple, scalable, and quantitative readout of protein-RNA interactions in vivo.

The study demonstrates that RBPscan can identify both linear and structured RNA-binding motifs, quantify relative binding affinities across multiple experimental systems including human cells, zebrafish embryos, and yeast, and link binding strength to functional outcomes such as mRNA decay. The method also enables precise mapping of binding sites within full-length transcripts, providing positional information that complements existing approaches.
By making quantitative analysis of protein-RNA interactions more accessible, RBPscan provides a versatile new tool for studying post-transcriptional gene regulation and opens the door to discovering how RNA-binding proteins function in their native biological contexts.
Congratulations to the exceptional and dedicated Cifuentes lab team who made this work possible, with Dmitry Kretov spearheading the experimental work and now leading his own lab at CHU de Québec-Université Laval Research Center.
Publisher online February 6th, 2026.
Links to the paper:
https://authors.elsevier.com/a/1mZlb3vVUPZNXj
https://www.cell.com/molecular-cell/fulltext/S1097-2765(26)00023-7
New research from the Grishok Lab: spermatogenic Argonaute proteins are activated by Insulin/IGF-1 Signaling and promote aging in C. elegans
New research from the Grishok Lab: spermatogenic Argonaute proteins are activated by Insulin/IGF-1 Signaling and promote aging in C. elegans

Congratulations to Stefan Isaac; one of the selected 2025 Toffler Scholars!
Congratulations to Stefan Isaac who was one of the selected 2025 Toffler Scholars!

The Toffler Scholar Program supports medical investigators engaged in early-stage, innovative medical research.
See announcement here:
Karen Toffler Charitable Trust Announces Toffler Scholars - Toffler Trust
New Research from the Layne Lab: mechanisms of adipose tissue fibrosis
A new study from the Layne laboratory identified a function for the transcription factor FOXS1 in regulating TGFb-dependent changes in adipogenic differentiation: JBC in press.
White adipose tissue (WAT) fibrosis is a major determinant of obesity-induced dysfunction and is characterized by excessive extracellular matrix deposition and myofibroblast activation. In this study, first author Alexander Tavares and co-authors identified FOXS1, a member of the forkhead box transcription factor superfamily, as a transcriptional target of TGF-β1 signaling in primary human adipocyte stem cells. FOXS1 also attenuated the induction of several adipogenic factors and sensitized cells to the anti-adipogenic effects of TGF-β1. Furthermore, loss of endogenous FOXS1 improved adipogenic permissiveness and activated proadipogenic gene programs in progenitors, even after TGF-β1 stimulation. These results indicate that FOXS1 is a positive regulator of profibrotic TGF-β1-dependent cellular responses, orchestrating the regulation of molecular phenotypes that promote myofibroblast activation and block adipogenesis. Co-authors in this study from the Layne lab include Scott Connelly, Daryn Maksat, Jane Zheng and Nabil Rabhi from the Farmer lab.
New news out of the Saeed Lab: Researchers Discover Protein Necessary for SARS-CoV-2 to Evade the Body’s Defenses
Congratulations to Barbara Schreiber being named educator of the year
Congratulations to Dr. Barbara Schreiber who has been named the 2025 Educator of the Year: PhD. 
New research out of the Lau Lab: Researchers Discover Protein Necessary for Fruit Fly Fertility
Researchers Discover Protein Necessary for Fruit Fly Fertility
The global birthrate has been in significant decline for decades. In the U.S., couples are deciding to have children later in life. A 2022 U.S. Census data analysis of Census Bureau and National Center for Health Statistics data, reveals that fertility rates for women age 20-24 declined by 43% during the period from 1990 to 2013. But the numbers of women age 35-39 giving birth increased by 67%, and for women between 40- 44 that increase was nearly 139%.
Women who decide to have children in middle age depend on sperm and egg resiliency. Part of germ cell (also known as egg cells and sperm cells) resilience depends on a functional piRNA pathway to protect germ cell genomes decades after puberty, which is when in humans, the Piwi pathway is activated as well as the expression of transposon RNAs—mobile DNA sequences that can move around a genome.

Researchers from Boston University Chobanian & Avedisian School of Medicine have found a new role for the transcription factor (proteins that regulate the transcription, or copying, of genes). In the fruit fly, this transcription factor, named Traffic Jam, activates a non-coding piRNA gene named Flamenco to promote female fruit fly (drosophila) fertility. The discovery solves the 30-year-old mystery of how Flamenco gets activated to protect fruit fly ovaries from a series of genetic parasites called retroviral transposons, and may one day help with infertility issues in humans.
“The discovery of Traffic Jam’s function in flies will help us look into humans with infertility symptoms and see if those patients lacking functional sperm may have defects in these Piwi genes and transcription factors,” explains corresponding author Nelson Lau, PhD, associate professor of biochemistry and director of the BU Genome Science Institute.

Lau and his colleagues first conducted a series of luciferase-reporter assays that measure gene activity and biological responses in 2017, discovering important new regulatory sequences in the Flamenco locus. They confirmed the biological importance of those Flamenco DNA sequences by generating new fruit fly mutants with CRISPR genome editing. They then carried out proteomics experiments and made the first discovery of Traffic Jam binding to Flamenco DNA sequences. Lastly, they conducted RNA interference knockdowns and chromatin immunoprecipitation sequencing studies of Traffic Jam in fruit fly ovary cells to confirm this new genetic interaction.
They found that Traffic Jam driving the production of Flamenco piRNAs bound by Piwi proteins, allowed fruit flies to protect their germline genome and produce fertile eggs and offspring. They also found that the retroviral transposons are also activated by Traffic Jam, hijacking this host factor. This finding is surprising because it demonstrates the on-going war between the animal’s genetic immune system and the genetic parasites fighting for their own survival.
According to the researchers, this study investigates the fundamental battle for the protection of germline genomes that humans depend on for fertility and reproduction. “We humans are like fruit flies in that our gonads also generate piRNAs to protect our germ cells against transposons. We have our own version of the Traffic Jam gene, called MAF-B, which we can test in future studies to see if MAF-B regulated human piRNA genes to allow us to produce functional sperm,” adds Lau.
These findings appear online in the journal Cell Reports. Lau’s team also contributed to an accompanying study in the same issue of Cell Reports by researchers in France and the United Kingdom.