A new paper led by Alex Luebbers in the Garcia-Marcos Lab dissects the molecular basis for how GINIP, a Gαi-interacting protein in neurons involved in controlling pain and seizures, modulates GPCR responses triggered by neurotransmitters. GINIP mimics how G-protein signaling effectors bind to Gα subunits via its PHD domain to differentially scale discrete G-protein signaling branches. The paper has been published in Structure (https://www.sciencedirect.com/science/article/abs/pii/S0969212623003891?via%3Dihub), and was previously posted on bioRxiv (https://www.biorxiv.org/content/10.1101/2023.04.20.537566v1).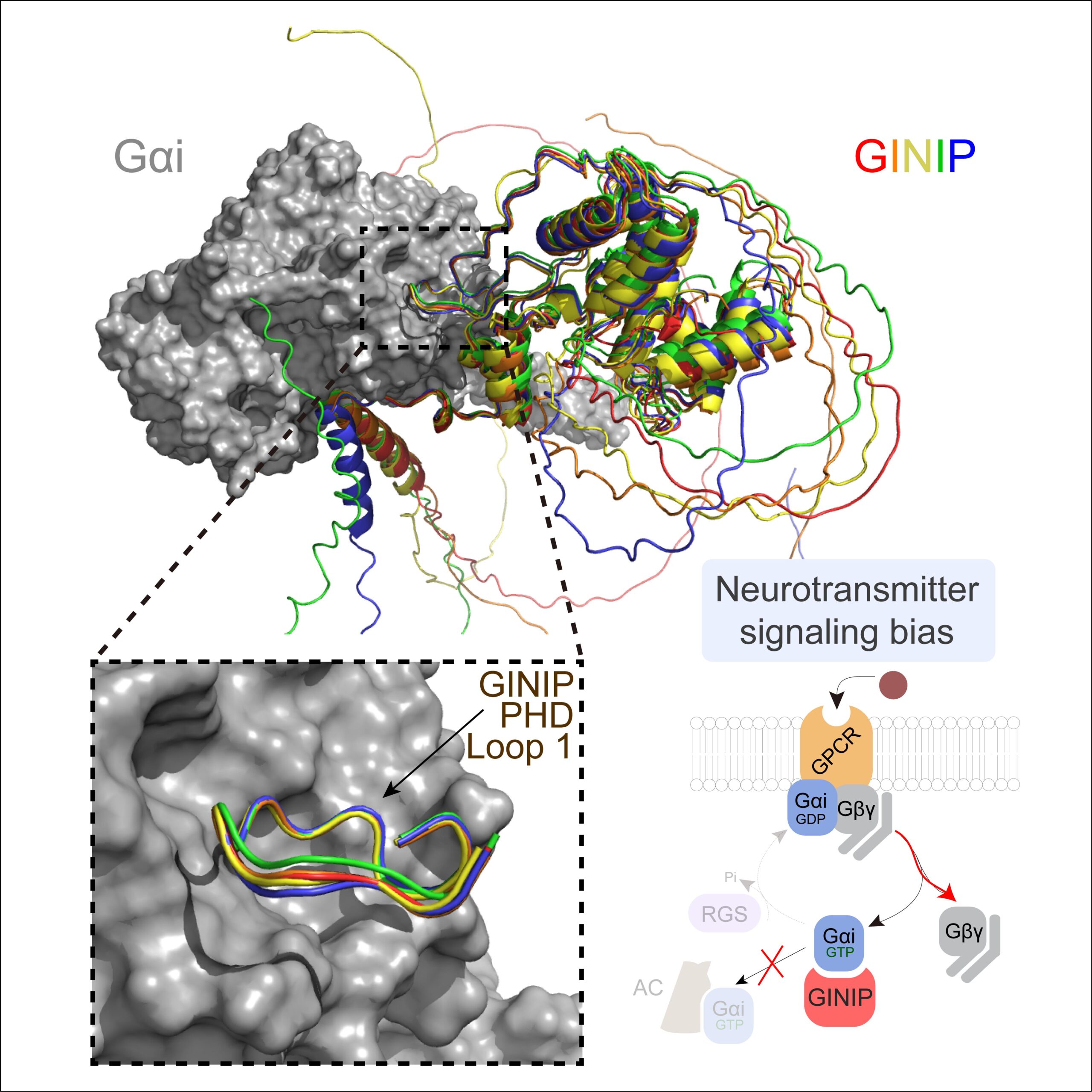
This paper builds and explands on another paper recently published in the same lab establishing that the neuronal protein GINIP shapes GPCR inhibitory neuromodulation via a unique mechanism of G-protein regulation that controls pain and seizure susceptibility (Park, Luebbers, et al Molecular Cell, 2023). However, the molecular basis of this mechanism remained ill-defined because the structural determinants of GINIP responsible for binding and regulating G proteins were not known. The newly published paper combined hydrogen-deuterium exchange mass spectrometry, computational structure predictions, biochemistry, and cell-based biophysical assays to demonstrate an effector-like binding mode of GINIP to Gαi. These findings explain the molecular basis for a post-receptor mechanism of G-protein regulation that fine-tunes inhibitory neuromodulation.
This paper features collaborations with the labs of Stephen Eyles at UMass-Amherst and of Joshua Levitz at Weill Cornell.