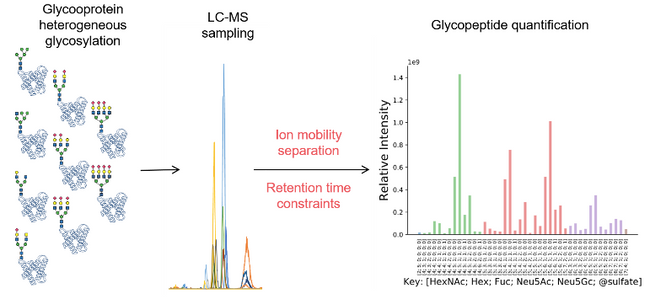
Most cell surface and extracellular matrix proteins are glycosylated as a mechanism driven by evolution that elaborates their adhesive interactions with binding partners. Such glycosylation occurs by biosynthetic reactions in the secretory pathway that gives rise to heterogeneity at each glycosylation site. This heterogeneity diversifies the range of interactions and therefore functions of glycoproteins. But, how can we understand the functional roles of glycoproteins if they are heterogeneous? Conventional proteomics methods have been adapted to quantify protein glycosylation heterogeneity, but understanding glycoprotein function requires improvements in sensitivity and selectivity. Chang and Zaia describe current methods of glycosylation quantification and show the promise of emerging glycoproteomics methods that employ data-independent analysis and ion mobility separations.