A new study from the Layne laboratory identified a novel mechanism for vascular adventitial remodeling. The paper, Aortic carboxypeptidase-like protein regulates vascular adventitial progenitor and fibroblast differentiation through myocardin related transcription factor A was published on February 17, 2021 in Scientific Reports.
The vascular adventitia, or outer layer of blood vessels contains numerous cell types including fibroblasts which are responsible for extracellular matrix production, adipocytes, inflammatory cells, and progenitor cells.
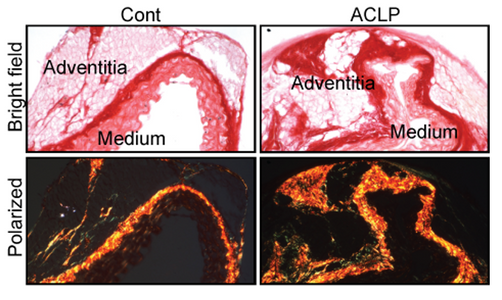 In response to vascular injury or disease adventitial firoblasts and progenitors become activated and secrete extracellular matrix proteins and become contractile leading to vessel remodeling. This study determined that the secreted protein Aortic carboxypeptidase-like protein (ACLP) repressed adventitial stem cell markers (CD34, KLF4) and increased collagen expression. Furthermore, ACLP enhanced the nuclear translocation of the transcriptional regulator myocardin-related transcription factor A (MRTFA) in adventitial cells. This study also determined that progenitor cells from MRTFA-null mice exhibited reduced smooth collagen and smooth muscle actin expression indicating that adventitial progenitor cell differentiation is regulated in part by the ACLP-MRTFA axis. Collectively these studies identified ACLP as a mediator of adventitial cellular differentiation, which may result in pathological vessel remodeling.
In response to vascular injury or disease adventitial firoblasts and progenitors become activated and secrete extracellular matrix proteins and become contractile leading to vessel remodeling. This study determined that the secreted protein Aortic carboxypeptidase-like protein (ACLP) repressed adventitial stem cell markers (CD34, KLF4) and increased collagen expression. Furthermore, ACLP enhanced the nuclear translocation of the transcriptional regulator myocardin-related transcription factor A (MRTFA) in adventitial cells. This study also determined that progenitor cells from MRTFA-null mice exhibited reduced smooth collagen and smooth muscle actin expression indicating that adventitial progenitor cell differentiation is regulated in part by the ACLP-MRTFA axis. Collectively these studies identified ACLP as a mediator of adventitial cellular differentiation, which may result in pathological vessel remodeling.
In addition to the first author Dahai Wang, collaborators on this project include Steve Farmer and Nabil Rabhi in the Department of Biochemistry and Shaw-Fang Yet from the Institute of Cellular and System Medicine in Taiwan.
This work was supported by NIH grants HL078869, DK117161, a grant from the American Heart Association, and the Evans Center for Interdisciplinary Research Fibrosis Affinity Research Collaborative.